Endothelial tissue remodeling induced by intraluminal pressure enhances paracellular solute transport
- PMID: 37416478
- PMCID: PMC10320514
- DOI: 10.1016/j.isci.2023.107141
Endothelial tissue remodeling induced by intraluminal pressure enhances paracellular solute transport
Abstract
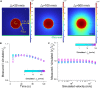
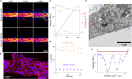
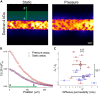
The endothelial layers of the microvasculature regulate the transport of solutes to the surrounding tissues. It remains unclear how this barrier function is affected by blood flow-induced intraluminal pressure. Using a 3D microvessel model, we compare the transport of macromolecules through endothelial tissues at mechanical rest or with intraluminal pressure, and correlate these data with electron microscopy of endothelial junctions. On application of an intraluminal pressure of 100 Pa, we demonstrate that the flow through the tissue increases by 2.35 times. This increase is associated with a 25% expansion of microvessel diameter, which leads to tissue remodeling and thinning of the paracellular junctions. We recapitulate these data with the deformable monopore model, in which the increase in paracellular transport is explained by the augmentation of the diffusion rate across thinned junctions under mechanical stress. We therefore suggest that the deformation of microvasculatures contributes to regulate their barrier function.
Keywords: Bioengineering; Biomechanics; Cell biology; Computer modeling; Tissue engineering.
© 2023 The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Intraluminal pressure triggers a rapid and persistent reinforcement of endothelial barriers.Lab Chip. 2025 Apr 8;25(8):2061-2072. doi: 10.1039/d5lc00104h. Lab Chip. 2025. PMID: 40099485
-
The transport of LDL across the deformable arterial wall: the effect of endothelial cell turnover and intimal deformation under hypertension.Am J Physiol Heart Circ Physiol. 2009 Sep;297(3):H983-96. doi: 10.1152/ajpheart.00324.2009. Epub 2009 Jul 10. Am J Physiol Heart Circ Physiol. 2009. PMID: 19592615 Free PMC article.
-
Regulation of endothelial junctional permeability.Ann N Y Acad Sci. 2008 Mar;1123:134-45. doi: 10.1196/annals.1420.016. Ann N Y Acad Sci. 2008. PMID: 18375586 Review.
-
Transcellular Pathways in Lymphatic Endothelial Cells Regulate Changes in Solute Transport by Fluid Stress.Circ Res. 2017 Apr 28;120(9):1440-1452. doi: 10.1161/CIRCRESAHA.116.309828. Epub 2017 Jan 27. Circ Res. 2017. PMID: 28130294
-
Endothelial cell barriers: Transport of molecules between blood and tissues.Traffic. 2019 Jun;20(6):390-403. doi: 10.1111/tra.12645. Epub 2019 Apr 24. Traffic. 2019. PMID: 30950163 Review.
Cited by
-
CD40 is expressed in the subsets of endothelial cells undergoing partial endothelial-mesenchymal transition in tumor microenvironment.Cancer Sci. 2024 Feb;115(2):490-506. doi: 10.1111/cas.16045. Epub 2023 Dec 18. Cancer Sci. 2024. PMID: 38111334 Free PMC article.
References
LinkOut - more resources
Full Text Sources

