A simple method for poly-D-lysine coating to enhance adhesion and maturation of primary cortical neuron cultures in vitro
- PMID: 37416506
- PMCID: PMC10320290
- DOI: 10.3389/fncel.2023.1212097
A simple method for poly-D-lysine coating to enhance adhesion and maturation of primary cortical neuron cultures in vitro
Abstract
Introduction: Glass coverslips are used as a substrate since Harrison's initial nerve cell culture experiments in 1910. In 1974, the first study of brain cells seeded onto polylysine (PL) coated substrate was published. Usually, neurons adhere quickly to PL coating. However, maintaining cortical neurons in culture on PL coating for a prolonged time is challenging.
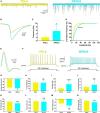
Methods: A collaborative study between chemical engineers and neurobiologists was conducted to find a simple method to enhance neuronal maturation on poly-D-lysine (PDL). In this work, a simple protocol to coat PDL efficiently on coverslips is presented, characterized, and compared to a conventional adsorption method. We studied the adhesion and maturation of primary cortical neurons with various morphological and functional approaches, including phase contrast microscopy, immunocytochemistry, scanning electron microscopy, patch clamp recordings, and calcium imaging.
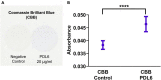
Results: We observed that several parameters of neuronal maturation are influenced by the substrate: neurons develop more dense and extended networks and synaptic activity is enhanced, when seeded on covalently bound PDL compared to adsorbed PDL.
Discussion: Hence, we established reproducible and optimal conditions enhancing maturation of primary cortical neurons in vitro. Our method allows higher reliability and yield of results and could also be profitable for laboratories using PL with other cell types.
Keywords: glass coverslip; neuronal adhesion; neuronal maturation; neuronal networks; poly-D-lysine grafting; primary cortical neuron cultures; synaptic activity; synaptic contacts.
Copyright © 2023 Stil, Liberelle, Guadarrama Bello, Lacomme, Arpin, Parent, Nanci, Dumont, Ould-Bachir, Vanni, De Crescenzo and Bouchard.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Bordoni R., Consolandi C., Castiglioni B., Busti E., Bernardi L. R., Battaglia C., et al. (2002). Investigation of the multiple anchors approach in oligonucleotide microarray preparation using linear and stem-loop structured probes. Nucleic Acids Res. 30:E34. 10.1093/nar/30.8.e34 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

