Assessment on the efficacy of methods 2 to 5 and method 7 set out in Commission Regulation (EU) No 142/2011 to inactivate relevant pathogens when producing processed animal protein of porcine origin intended to feed poultry and aquaculture animals
- PMID: 37416785
- PMCID: PMC10320699
- DOI: 10.2903/j.efsa.2023.8093
Assessment on the efficacy of methods 2 to 5 and method 7 set out in Commission Regulation (EU) No 142/2011 to inactivate relevant pathogens when producing processed animal protein of porcine origin intended to feed poultry and aquaculture animals
Abstract
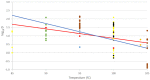
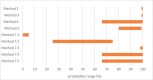
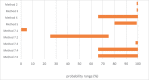
An assessment was conducted on the level of inactivation of relevant pathogens that could be present in processed animal protein of porcine origin intended to feed poultry and aquaculture animals when methods 2 to 5 and method 7, as detailed in Regulation (EU) No 142/2011, are applied. Five approved scenarios were selected for method 7. Salmonella Senftenberg, Enterococcus faecalis, spores of Clostridium perfringens and parvoviruses were shortlisted as target indicators. Inactivation parameters for these indicators were extracted from extensive literature search and a recent EFSA scientific opinion. An adapted Bigelow model was fitted to retrieved data to estimate the probability that methods 2 to 5, in coincidental and consecutive modes, and the five scenarios of method 7 are able to achieve a 5 log10 and a 3 log10 reduction of bacterial indicators and parvoviruses, respectively. Spores of C. perfringens were the indicator with the lowest probability of achieving the target reduction by methods 2 to 5, in coincidental and consecutive mode, and by the five considered scenarios of method 7. An expert knowledge elicitation was conducted to estimate the certainty of achieving a 5 log10 reduction of spores of C. perfringens considering the results of the model and additional evidence. A 5 log10 reduction of C. perfringens spores was judged: 99-100% certain for methods 2 and 3 in coincidental mode; 98-100% certain for method 7 scenario 3; 80-99% certain for method 5 in coincidental mode; 66-100% certain for method 4 in coincidental mode and for method 7 scenarios 4 and 5; 25-75% certain for method 7 scenario 2; and 0-5% certain for method 7 scenario 1. Higher certainty is expected for methods 2 to 5 in consecutive mode compared to coincidental mode.
Keywords: animal by‐products; feed; inactivation; pathogens; perfringens; porcine.
© 2023 European Food Safety Authority. EFSA Journal published by Wiley‐VCH GmbH on behalf of European Food Safety Authority.
Figures














References
-
- Abworo EO, Onzere C, Amimo JO, Riitho V, Mwangi W, Davies J, Blome S and Bishop RP, 2017. Detection of African swine fever virus in the tissues of asymptomatic pigs in smallholder farming systems along the Kenya‐Uganda border: implications for transmission in endemic areas and ASF surveillance in East Africa. Journal of General Virology, 98, 1806–1814. - PubMed
-
- Adedeji AJ, Atai RB, Gyang HE, Gambo P, Habib MA, Weka R, Muwanika VB, Masembe C and Luka PD, 2022. Live pig markets are hotspots for spread of African swine fever virus in Nigeria. Transboundary and Emerging Diseases, 69, e1526–e1540. - PubMed
-
- Almeida MN, Zimmerman JJ, Wang C and Linhares DCL, 2018. Assessment of abattoir based monitoring of PRRSV using oral fluids. Preventive Veterinary Medicine, 158, 137–145. - PubMed
-
- Amorim AR, Fornells L, Reis FD, Rezende DJ, Mendes GD, Couceiro J and Santos NSD, 2013. Influenza A virus infection of healthy piglets in an abattoir in Brazil: animal‐human interface and risk for interspecies transmission. Memorias Do Instituto Oswaldo Cruz, 108, 548–553. 10.1590/0074-0276108052013003 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
