Production of Dipeptidyl Peptidase-IV Inhibitory Peptides from Germinated Jack Bean [ Canavalia ensiformis (L.) DC.] Flour
- PMID: 37416790
- PMCID: PMC10321440
- DOI: 10.3746/pnf.2023.28.2.149
Production of Dipeptidyl Peptidase-IV Inhibitory Peptides from Germinated Jack Bean [ Canavalia ensiformis (L.) DC.] Flour
Abstract
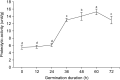
An alternate plant-based protein, jack bean sprout, was explored as a source of bioactive peptides. Germination to increase dipeptidyl peptidase-IV (DPP-IV) inhibitory peptide in jack bean sprout flour has yet to be reported. Therefore, this study aimed to investigate the optimal condition to maximize the content of bioactive peptides with maximum DPP-IV inhibitory activity. The relationship between germination and DPP-IV inhibitory activity was determined by analyzing the proteolytic activity, percentage of degree of hydrolysis (%DH), and peptide content. Peptide samples with the most potent DPP-IV inhibitory activity were subsequently fractionated, identified, and characterized. The 60-h germinated jack bean had the best DPP-IV inhibitory activity (41.57%; half maximal inhibitory concentration=2.24 mg/mL). Proteolytic activity (15.24 unit/g), %DH (11.43%), and peptide content (59.71 mg/g) supported this result. Furthermore, the <1.0 kDa peptide fraction of this sprouted flour had the highest molecular weight (MW) distribution (32.60%) and DPP-IV inhibitory activity (71.99%). Peptide sequences identified from MW <1.0 and 1.0∼3.5 kDa peptide fractions had valine, leucine, isoleucine, glycine, and tryptophan at the N-terminal and also had alanine at the penultimate N-terminal, verifying their presence as DPP-IV inhibitors. Furthermore, peptide sequences generated exhibited other biological activities, including angiotensin-converting enzyme, renin, and α-glucosidase inhibitors.
Keywords: dipeptidyl peptidase-IV inhibitors; germination; jack bean sprout flour; peptide fraction; peptide sequence.
Copyright © 2023 by The Korean Society of Food Science and Nutrition. All rights Reserved.
Conflict of interest statement
AUTHOR DISCLOSURE STATEMENT The authors declare no conflict of interest.
Figures




References
-
- Afify Ael-M, El-Beltagi HS, El-Salam SM, Omran AA. Protein solubility, digestibility and fractionation after germination of sorghum varieties. PLoS One. 2012;7:e31154. doi: 10.1371/journal.pone.0031154. https://doi.org/10.1371/journal.pone.0031154. - DOI - PMC - PubMed
-
- BIOPEP-UWM database. 2022. [[cited 2022 Oct 10]]. Available from: https://biochemia.uwm.edu.pl/biopep-uwm/
-
- Botcha S, Prattipati SD. Role of amylase and protease in germinating Sterculia urens Roxb. Bangladesh J Sci Ind Res. 2020;55:107–112. doi: 10.3329/bjsir.v55i2.47631. - DOI
-
- Chang KC, Skauge LH, Satterlee LD. Analysis of amino acids in soy isolates and navy beans using precolumn derivatization with phenylisothiocyanate and reversed-phase high performance liquid chromatography. J Food Sci. 1989;54:756–757. doi: 10.1111/j.1365-2621.1989.tb04699.x. - DOI
-
- Chauhan A, Kumari N, Saxena DC, Singh S. Effect of germination on fatty acid profile, amino acid profile and minerals of amaranth (Amaranthus spp.) grain. Food Meas. 2022;16:1777–1786. doi: 10.1007/s11694-022-01292-7. - DOI
LinkOut - more resources
Full Text Sources
