Upconversion nanoparticle-based aptasensor for rapid and ultrasensitive detection of Staphylococcus aureus by low-speed centrifugation
- PMID: 37416905
- PMCID: PMC10321366
- DOI: 10.1039/d3ra01555f
Upconversion nanoparticle-based aptasensor for rapid and ultrasensitive detection of Staphylococcus aureus by low-speed centrifugation
Abstract
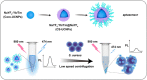
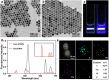
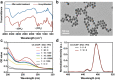
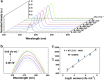
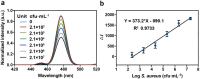
Opportunistic foodborne pathogens such as Staphylococcus aureus (S. aureus) can cause a wide variety of threats to public health. There is an urgent clinical need for a fast, simple, low-cost, and sensitive method. Here, we designed a fluorescence-based aptamer biosensor (aptasensor) for S. aureus detection using core-shell structured upconversion nanoparticles (CS-UCNPs) as a beacon. A S. aureus-specific aptamer was modified on the surface of CS-UCNPs for binding pathogens. The S. aureus bound to CS-UCNPs can then be isolated from the detection system by simple low-speed centrifugation. Thus, an aptasensor was successfully established for the detection of S. aureus. The fluorescence intensity of CS-UCNPs correlated with the concentration of S. aureus within the range of 6.36 × 102 to 6.36 × 108 CFU mL-1, resulting in the detected limit of S. aureus being 60 CFU mL-1. The aptasensor performed well in real food samples (milk) with a detection limit of 146 CFU mL-1 for S. aureus. Furthermore, we applied our aptasensor in chicken muscles for S. aureus detection, and compared it with the plate count gold standard method. There was no significant difference between our aptasensor and the plate count method within the detected limit, while the time for the aptasensor (0.58 h) was shorter than that of the plate count method (3-4 d). Therefore, we succeeded in the design of a simple, sensitive and fast CS-UCNPs aptasensor for S. aureus detection. This aptasensor system would have the potential for the detection of a wide range of bacterial species by switching the corresponding aptamer.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Upconversion nanoparticles-based FRET system for sensitive detection of Staphylococcus aureus.Spectrochim Acta A Mol Biomol Spectrosc. 2021 Jul 5;255:119734. doi: 10.1016/j.saa.2021.119734. Epub 2021 Mar 23. Spectrochim Acta A Mol Biomol Spectrosc. 2021. PMID: 33812237
-
Ultrasensitive hairpin mediated upconversion fluorescence biosensor for Staphylococcus aureus detection in foods and waters exploiting g-C3N4-assisted catalysis.Anal Chim Acta. 2023 Jan 25;1239:340738. doi: 10.1016/j.aca.2022.340738. Epub 2022 Dec 21. Anal Chim Acta. 2023. PMID: 36628775
-
An ultrasensitive electrochemical aptasensor using Tyramide-assisted enzyme multiplication for the detection of Staphylococcus aureus.Biosens Bioelectron. 2023 May 15;228:115199. doi: 10.1016/j.bios.2023.115199. Epub 2023 Mar 8. Biosens Bioelectron. 2023. PMID: 36906992
-
Upconversion nanoparticles based FRET aptasensor for rapid and ultrasenstive bacteria detection.Biosens Bioelectron. 2017 Apr 15;90:525-533. doi: 10.1016/j.bios.2016.10.029. Epub 2016 Oct 19. Biosens Bioelectron. 2017. PMID: 27825886
-
Multiple amplification-based fluorometric aptasensor for highly sensitive detection of Staphylococcus aureus.Appl Microbiol Biotechnol. 2022 Oct;106(19-20):6733-6743. doi: 10.1007/s00253-022-12057-z. Epub 2022 Sep 5. Appl Microbiol Biotechnol. 2022. PMID: 36058939
References
-
- Zhao X. Lin C.-W. Wang J. Oh D. H. J. Microbiol. Biotechnol. 2014;24:297–312. - PubMed
-
- Martinon A. Wilkinson M. J. Food Saf. 2011;31:297–312.
-
- Samal S. K. Dash M. Declercq H. A. Gheysens T. Dendooven J. Van der Voort P. Cornelissen R. Dubruel P. Kaplan D. L. Macromol. Biosci. 2014;14:991–1003. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

