Distinct Transcriptional and Functional Differences of Lung Resident and Monocyte-Derived Alveolar Macrophages During the Recovery Period of Acute Lung Injury
- PMID: 37416929
- PMCID: PMC10320419
- DOI: 10.4110/in.2023.23.e24
Distinct Transcriptional and Functional Differences of Lung Resident and Monocyte-Derived Alveolar Macrophages During the Recovery Period of Acute Lung Injury
Abstract
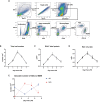
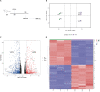
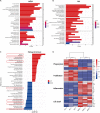
In acute lung injury, two subsets of lung macrophages exist in the alveoli: tissue-resident alveolar macrophages (AMs) and monocyte-derived alveolar macrophages (MDMs). However, it is unclear whether these 2 subsets of macrophages have different functions and characteristics during the recovery phase. RNA-sequencing of AMs and MDMs from the recovery period of LPS-induced lung injury mice revealed their differences in proliferation, cell death, phagocytosis, inflammation and tissue repair. Using flow cytometry, we found that AMs showed a higher ability to proliferate, whereas MDMs expressed a larger amount of cell death. We also compared the ability of phagocytosing apoptotic cells and activating adaptive immunity and found that AMs have a stronger ability to phagocytose, while MDMs are the cells that activate lymphocytes during the resolving phase. By testing surface markers, we found that MDMs were more prone to the M1 phenotype, but expressed a higher level of pro-repairing genes. Finally, analysis of a publicly available set of single-cell RNA-sequencing data on bronchoalveolar lavage cells from patients with SARS-CoV-2 infection validated the double-sided role of MDMs. Blockade of inflammatory MDM recruitment using CCR2-/- mice effectively attenuates lung injury. Therefore, AMs and MDMs exhibited large differences during recovery. AMs are long-lived M2-like tissue-resident macrophages that have a strong ability to proliferate and phagocytose. MDMs are a paradoxical group of macrophages that promote the repair of tissue damage despite being strongly pro-inflammatory early in infection, and they may undergo cell death as inflammation fades. Preventing the massive recruitment of inflammatory MDMs or promoting their transition to pro-repairing phenotype may be a new direction for the treatment of acute lung injury.
Keywords: Acute Lung Injury; Alveolar Macrophage; Inflammation; Monocyte-Derived Macrophage.
Copyright © 2023. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures



Similar articles
-
Monocyte-Derived Macrophages Induce Alveolar Macrophages Death via TNF-α in Acute Lung Injury.Immun Inflamm Dis. 2024 Dec;12(12):e70081. doi: 10.1002/iid3.70081. Immun Inflamm Dis. 2024. PMID: 39660881 Free PMC article.
-
RAMP1 Signaling Mitigates Acute Lung Injury by Distinctively Regulating Alveolar and Monocyte-Derived Macrophages.Int J Mol Sci. 2024 Sep 20;25(18):10107. doi: 10.3390/ijms251810107. Int J Mol Sci. 2024. PMID: 39337592 Free PMC article.
-
Monocyte-Derived Macrophages Contribute to Spontaneous Long-Term Functional Recovery after Stroke in Mice.J Neurosci. 2016 Apr 13;36(15):4182-95. doi: 10.1523/JNEUROSCI.4317-15.2016. J Neurosci. 2016. PMID: 27076418 Free PMC article.
-
The guardians of pulmonary harmony: alveolar macrophages orchestrating the symphony of lung inflammation and tissue homeostasis.Eur Respir Rev. 2024 May 29;33(172):230263. doi: 10.1183/16000617.0263-2023. Print 2024 Apr 30. Eur Respir Rev. 2024. PMID: 38811033 Free PMC article. Review.
-
Alveolar macrophages in lung cancer: opportunities challenges.Front Immunol. 2023 Sep 26;14:1268939. doi: 10.3389/fimmu.2023.1268939. eCollection 2023. Front Immunol. 2023. PMID: 37822933 Free PMC article. Review.
Cited by
-
Sphingosylphosphorylcholine Promotes Th9 Cell Differentiation Through Regulation of Smad3, STAT5, and β-Catenin Pathways.Immune Netw. 2024 Dec 26;24(6):e45. doi: 10.4110/in.2024.24.e45. eCollection 2024 Dec. Immune Netw. 2024. PMID: 39801737 Free PMC article.
-
Friend or foe: the role of platelets in acute lung injury.Front Immunol. 2025 May 14;16:1556923. doi: 10.3389/fimmu.2025.1556923. eCollection 2025. Front Immunol. 2025. PMID: 40438116 Free PMC article. Review.
-
Single-cell transcriptome analysis of the mouse lungs during the injury and recovery periods after lipopolysaccharide administration.Inflamm Res. 2024 Dec;73(12):2087-2107. doi: 10.1007/s00011-024-01951-z. Epub 2024 Oct 8. Inflamm Res. 2024. PMID: 39377802
-
Targeting alveolar macrophages: a promising intervention for pulmonary infection and acute lung injury.Cell Mol Biol Lett. 2025 Jun 14;30(1):69. doi: 10.1186/s11658-025-00750-6. Cell Mol Biol Lett. 2025. PMID: 40517224 Free PMC article. Review.
-
Optimized method for higher yield of alveolar macrophage isolation for ex vivo studies.Heliyon. 2024 Aug 30;10(17):e37221. doi: 10.1016/j.heliyon.2024.e37221. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39319125 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

