NLRP3 Exacerbate NETosis-Associated Neuroinflammation in an LPS-Induced Inflamed Brain
- PMID: 37416934
- PMCID: PMC10320420
- DOI: 10.4110/in.2023.23.e27
NLRP3 Exacerbate NETosis-Associated Neuroinflammation in an LPS-Induced Inflamed Brain
Abstract
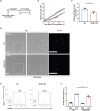
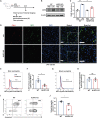
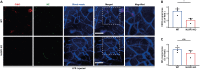
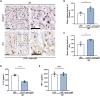
Neutrophil extracellular traps (NETs) exert a novel function of trapping pathogens. Released NETs can accumulate in inflamed tissues, be recognized by other immune cells for clearance, and lead to tissue toxicity. Therefore, the deleterious effect of NET is an etiological factor, causing several diseases directly or indirectly. NLR family pyrin domain containing 3 (NLRP3) in neutrophils is pivotal in signaling the innate immune response and is associated with several NET-related diseases. Despite these observations, the role of NLRP3 in NET formation in neuroinflammation remains elusive. Therefore, we aimed to explore NET formation promoted by NLRP3 in an LPS-induced inflamed brain. Wild-type and NLRP3 knockout mice were used to investigate the role of NLRP3 in NET formation. Brain inflammation was systemically induced by administering LPS. In such an environment, the NET formation was evaluated based on the expression of its characteristic indicators. DNA leakage and NET formation were analyzed in both mice through Western blot, flow cytometry, and in vitro live cell imaging as well as two-photon imaging. Our data revealed that NLRP3 promotes DNA leakage and facilitates NET formation accompanied by neutrophil death. Moreover, NLRP3 is not involved in neutrophil infiltration but is predisposed to boost NET formation, which is accompanied by neutrophil death in the LPS-induced inflamed brain. Furthermore, either NLRP3 deficiency or neutrophil depletion diminished pro-inflammatory cytokine, IL-1β, and alleviated blood-brain barrier damage. Overall, the results suggest that NLRP3 exacerbates NETosis in vitro and in the inflamed brain, aggravating neuroinflammation. These findings provide a clue that NLRP3 would be a potential therapeutic target to alleviate neuroinflammation.
Keywords: Brain; Inflammation; Intravital microscopy; NLR family pyrin domain-containing 3 protein; Neutrophil extracellular traps.
Copyright © 2023. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures



Similar articles
-
NLRP3 exacerbates EAE severity through ROS-dependent NET formation in the mouse brain.Cell Commun Signal. 2024 Feb 2;22(1):96. doi: 10.1186/s12964-023-01447-z. Cell Commun Signal. 2024. PMID: 38308301 Free PMC article.
-
Increased neutrophil extracellular traps activate NLRP3 and inflammatory macrophages in adult-onset Still's disease.Arthritis Res Ther. 2019 Jan 7;21(1):9. doi: 10.1186/s13075-018-1800-z. Arthritis Res Ther. 2019. PMID: 30616678 Free PMC article.
-
NLRP3 Inflammasome Assembly in Neutrophils Is Supported by PAD4 and Promotes NETosis Under Sterile Conditions.Front Immunol. 2021 May 28;12:683803. doi: 10.3389/fimmu.2021.683803. eCollection 2021. Front Immunol. 2021. PMID: 34122445 Free PMC article.
-
NLRP3-Induced NETosis: A Potential Therapeutic Target for Ischemic Thrombotic Diseases?Cells. 2023 Nov 26;12(23):2709. doi: 10.3390/cells12232709. Cells. 2023. PMID: 38067137 Free PMC article. Review.
-
Neutrophil Extracellular Traps Exacerbate Ischemic Brain Damage.Mol Neurobiol. 2022 Jan;59(1):643-656. doi: 10.1007/s12035-021-02635-z. Epub 2021 Nov 8. Mol Neurobiol. 2022. PMID: 34748205 Review.
Cited by
-
Peripheral endotoxin exposure in mice activates crosstalk between phagocytes in the brain and periphery.Res Sq [Preprint]. 2024 Jun 7:rs.3.rs-4478250. doi: 10.21203/rs.3.rs-4478250/v1. Res Sq. 2024. PMID: 38883776 Free PMC article. Preprint.
-
A Pilot Study to Investigate Peripheral Low-Level Chronic LPS Injection as a Model of Neutrophil Activation in the Periphery and Brain in Mice.Int J Mol Sci. 2024 May 14;25(10):5357. doi: 10.3390/ijms25105357. Int J Mol Sci. 2024. PMID: 38791393 Free PMC article.
-
Roles of the Caspase-11 Non-Canonical Inflammasome in Rheumatic Diseases.Int J Mol Sci. 2024 Feb 8;25(4):2091. doi: 10.3390/ijms25042091. Int J Mol Sci. 2024. PMID: 38396768 Free PMC article. Review.
-
The lactate metabolism and protein lactylation in epilepsy.Front Cell Neurosci. 2025 Jan 14;18:1464169. doi: 10.3389/fncel.2024.1464169. eCollection 2024. Front Cell Neurosci. 2025. PMID: 39876842 Free PMC article. Review.
-
Microfluidics-free single-cell genomics reveals complex central-peripheral immune crosstalk in the mouse brain during peripheral inflammation.Res Sq [Preprint]. 2023 Oct 17:rs.3.rs-3428910. doi: 10.21203/rs.3.rs-3428910/v1. Res Sq. 2023. PMID: 37886510 Free PMC article. Preprint.
References
-
- Hyun YM, Hong CW. Deep insight into neutrophil trafficking in various organs. J Leukoc Biol. 2017;102:617–629. - PubMed
-
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. - PubMed
-
- Saffarzadeh M. Neutrophil extracellular traps as a drug target to counteract chronic and acute inflammation. Curr Pharm Biotechnol. 2018;19:1196–1202. - PubMed
LinkOut - more resources
Full Text Sources

