Platelet-Rich Plasma Injections for the Treatment of Ankle Osteoarthritis
- PMID: 37417359
- PMCID: PMC10394962
- DOI: 10.1177/03635465231182438
Platelet-Rich Plasma Injections for the Treatment of Ankle Osteoarthritis
Abstract
Background: Ankle osteoarthritis is debilitating and usually affects relatively young people, often as a result of previous ankle traumas, frequently occurring in sports. Platelet-rich plasma (PRP) injections for ankle osteoarthritis have shown no evidence of benefit over the course of 26 weeks. Previous studies on PRP for knee osteoarthritis showed that clinically significant improvements with PRP occurred between 6 to 12 months in the absence of initial benefit. No studies have evaluated the effect of PRP from 6 to 12 months in ankle osteoarthritis.
Purpose: To assess the efficacy of PRP injections in ankle osteoarthritis over the course of 52 weeks.
Study design: Randomized controlled trial; Level of evidence, 1.
Methods: In this 52-week follow-up trial, 100 patients with ankle osteoarthritis were randomized to a PRP group or placebo (saline) group. Patients received 2 intra-articular talocrural injections: at inclusion and after 6 weeks. Patient-reported outcome measures were used to assess pain, function, quality of life, and indirect costs over 52 weeks.
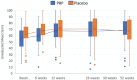
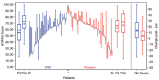
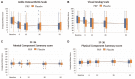
Results: Two patients (2%) were lost to follow-up. The adjusted between-group difference for the patient-reported American Orthopaedic Foot & Ankle Society score over 52 weeks was -2 points (95% CI, -5 to 2; P = .31) in favor of the placebo group. No significant between-group differences were observed for any of the secondary outcome measures.
Conclusion: For patients with ankle osteoarthritis, PRP injections did not improve ankle symptoms and function over 52 weeks compared with placebo injections.
Registration: NTR7261 (Netherlands Trial Register).
Keywords: ankle osteoarthritis; orthopaedic sports medicine; osteoarthritis; platelet-rich plasma; sports medicine.
Conflict of interest statement
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was supported by a grant from the Dutch Arthritis Society. The Dutch Arthritis Society (a nonprofit patient organization) had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The platelet-rich plasma centrifugation system was on loan from Arthrex (Arthrex Medizinische Instrumente GmbH), as is customary under usual care. G.R. has received research support from Arthrex. A.W. has received research support from Arthrex and Biomet. S.M.A.B.-Z. has received consulting fees from Pfizer. G.M.M.J.K. has received research support from Arthrex. J.L.T. has received research support from Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Figures
Comment in
-
Improving Injectable Orthobiologics Reporting Guidelines Adherence: Letter to the Editor.Am J Sports Med. 2023 Dec;51(14):NP66. doi: 10.1177/03635465231203205. Am J Sports Med. 2023. PMID: 38031746 No abstract available.
-
Platelet-Rich Plasma Injections for the Treatment of Ankle Osteoarthritis: Letter to the Editor.Am J Sports Med. 2024 Feb;52(2):NP3. doi: 10.1177/03635465231213857. Am J Sports Med. 2024. PMID: 38305235 No abstract available.
References
-
- Aaronson NK, Muller M, Cohen PDA, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51(11):1055-1068. - PubMed
-
- Angthong C, Khadsongkram A, Angthong W. Outcomes and quality of life after platelet-rich plasma therapy in patients with recalcitrant hindfoot and ankle diseases: a preliminary report of 12 patients. J Foot Ankle Surg. 2013;52(4):475-480. - PubMed
-
- Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Roijen LH van. The iMTA Productivity Cost Questionnaire. Value Health. 2015;18(6):753-758. - PubMed
-
- Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

