Yin Yang 1 controls cerebellar astrocyte maturation
- PMID: 37417428
- PMCID: PMC10529878
- DOI: 10.1002/glia.24434
Yin Yang 1 controls cerebellar astrocyte maturation
Abstract
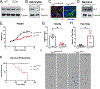
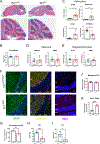
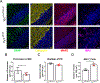
Diverse subpopulations of astrocytes tile different brain regions to accommodate local requirements of neurons and associated neuronal circuits. Nevertheless, molecular mechanisms governing astrocyte diversity remain mostly unknown. We explored the role of a zinc finger transcription factor Yin Yang 1 (YY1) that is expressed in astrocytes. We found that specific deletion of YY1 from astrocytes causes severe motor deficits in mice, induces Bergmann gliosis, and results in simultaneous loss of GFAP expression in velate and fibrous cerebellar astrocytes. Single cell RNA-seq analysis showed that YY1 exerts specific effects on gene expression in subpopulations of cerebellar astrocytes. We found that although YY1 is dispensable for the initial stages of astrocyte development, it regulates subtype-specific gene expression during astrocyte maturation. Moreover, YY1 is continuously needed to maintain mature astrocytes in the adult cerebellum. Our findings suggest that YY1 plays critical roles regulating cerebellar astrocyte maturation during development and maintaining a mature phenotype of astrocytes in the adult cerebellum.
Keywords: YY1; astrocytes; cerebellum; heterogeneity; maturation.
© 2023 The Authors. GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

