Pharmacological, non-invasive brain stimulation and psychological interventions, and their combination, for treating depression after stroke
- PMID: 37417452
- PMCID: PMC10327406
- DOI: 10.1002/14651858.CD003437.pub5
Pharmacological, non-invasive brain stimulation and psychological interventions, and their combination, for treating depression after stroke
Abstract
Background: Depression is an important morbidity associated with stroke that impacts on recovery, yet is often undetected or inadequately treated.
Objectives: To evaluate the benefits and harms of pharmacological intervention, non-invasive brain stimulation, psychological therapy, or combinations of these to treat depression after stroke.
Search methods: This is a living systematic review. We search for new evidence every two months and update the review when we identify relevant new evidence. Please refer to the Cochrane Database of Systematic Reviews for the current status of this review. We searched the Specialised Registers of Cochrane Stroke, and Cochrane Depression Anxiety and Neurosis, CENTRAL, MEDLINE, Embase, five other databases, two clinical trials registers, reference lists and conference proceedings (February 2022). We contacted study authors.
Selection criteria: Randomised controlled trials (RCTs) comparing: 1) pharmacological interventions with placebo; 2) non-invasive brain stimulation with sham stimulation or usual care; 3) psychological therapy with usual care or attention control; 4) pharmacological intervention and psychological therapy with pharmacological intervention and usual care or attention control; 5) pharmacological intervention and non-invasive brain stimulation with pharmacological intervention and sham stimulation or usual care; 6) non-invasive brain stimulation and psychological therapy versus sham brain stimulation or usual care and psychological therapy; 7) pharmacological intervention and psychological therapy with placebo and psychological therapy; 8) pharmacological intervention and non-invasive brain stimulation with placebo and non-invasive brain stimulation; and 9) non-invasive brain stimulation and psychological therapy versus non-invasive brain stimulation and usual care or attention control, with the intention of treating depression after stroke.
Data collection and analysis: Two review authors independently selected studies, assessed risk of bias, and extracted data from included studies. We calculated mean difference (MD) or standardised mean difference (SMD) for continuous data, and risk ratio (RR) for dichotomous data, with 95% confidence intervals (CIs). We assessed heterogeneity using the I² statistic and certainty of the evidence according to GRADE.
Main results: We included 65 trials (72 comparisons) with 5831 participants. Data were available for: 1) 20 comparisons; 2) nine comparisons; 3) 25 comparisons; 4) three comparisons; 5) 14 comparisons; and 6) one comparison. We found no trials for comparisons 7 to 9. Comparison 1: Pharmacological interventions Very low-certainty evidence from eight trials suggests pharmacological interventions decreased the number of people meeting the study criteria for depression (RR 0.70, 95% CI 0.55 to 0.88; P = 0.002; 8 RCTs; 1025 participants) at end of treatment and very low-certainty evidence from six trials suggests that pharmacological interventions decreased the number of people with inadequate response to treatment (RR 0.47, 95% CI 0.32 to 0.70; P = 0.0002; 6 RCTs; 511 participants) compared to placebo. More adverse events related to the central nervous system (CNS) (RR 1.55, 95% CI 1.12 to 2.15; P = 0.008; 5 RCTs; 488 participants; very low-certainty evidence) and gastrointestinal system (RR 1.62, 95% CI 1.19 to 2.19; P = 0.002; 4 RCTs; 473 participants; very low-certainty evidence) were noted in the pharmacological intervention than in the placebo group. Comparison 2: Non-invasive brain stimulation Very low-certainty evidence from two trials show that non-invasive brain stimulation had little to no effect on the number of people meeting the study criteria for depression (RR 0.67, 95% CI 0.39 to 1.14; P = 0.14; 2 RCTs; 130 participants) and the number of people with inadequate response to treatment (RR 0.84, 95% CI 0.52, 1.37; P = 0.49; 2 RCTs; 130 participants) compared to sham stimulation. Non-invasive brain stimulation resulted in no deaths. Comparison 3: Psychological therapy Very low-certainty evidence from six trials suggests that psychological therapy decreased the number of people meeting the study criteria for depression at end of treatment (RR 0.77, 95% CI 0.62 to 0.95; P = 0.01; 521 participants) compared to usual care/attention control. No trials of psychological therapy reported on the outcome inadequate response to treatment. No differences in the number of deaths or adverse events were found in the psychological therapy group compared to the usual care/attention control group. Comparison 4: Pharmacological interventions with psychological therapy No trials of this combination reported on the primary outcomes. Combination therapy resulted in no deaths. Comparison 5: Pharmacological interventions with non-invasive brain stimulation Non-invasive brain stimulation with pharmacological intervention reduced the number of people meeting study criteria for depression at end of treatment (RR 0.77, 95% CI 0.64 to 0.91; P = 0.002; 3 RCTs; 392 participants; low-certainty evidence) but not the number of people with inadequate response to treatment (RR 0.95, 95% CI 0.69 to 1.30; P = 0.75; 3 RCTs; 392 participants; very low-certainty evidence) compared to pharmacological therapy alone. Very low-certainty evidence from five trials suggest no difference in deaths between this combination therapy (RR 1.06, 95% CI 0.27 to 4.16; P = 0.93; 487 participants) compared to pharmacological therapy intervention and sham stimulation or usual care. Comparison 6: Non-invasive brain stimulation with psychological therapy No trials of this combination reported on the primary outcomes.
Authors' conclusions: Very low-certainty evidence suggests that pharmacological, psychological and combination therapies can reduce the prevalence of depression while non-invasive brain stimulation had little to no effect on the prevalence of depression. Pharmacological intervention was associated with adverse events related to the CNS and the gastrointestinal tract. More research is required before recommendations can be made about the routine use of such treatments.
Antecedentes: La depresión tiene una morbilidad importante asociada con el accidente cerebrovascular que repercute en la recuperación, pero que a menudo no se detecta o se trata de manera inadecuada.
Objetivos: Evaluar los efectos beneficiosos y perjudiciales de las intervenciones farmacológicas, la estimulación cerebral no invasiva, la terapia psicológica o las combinaciones de éstas para tratar la depresión después del accidente cerebrovascular. MÉTODOS DE BÚSQUEDA: Esta es una revisión sistemática continua. Cada dos meses se busca nueva evidencia y la revisión se actualiza cuando se identifica evidencia nueva relevante. Consultar el estado actual de esta revisión en la Base de Datos Cochrane de Revisiones Sistemáticas (Cochrane Database of Systematic Reviews). Se realizaron búsquedas en los Registros especializados del Grupo Cochrane de Accidentes cerebrovasculares (Cochrane Stroke) y del Grupo Cochrane de Depresión, ansiedad y neurosis (Cochrane Depression, Anxiety and Neurosis), en CENTRAL, MEDLINE, Embase, otras cinco bases de datos, dos registros de ensayos clínicos, listas de referencias y resúmenes de congresos (febrero de 2022). Se estableció contacto con autores de estudios. CRITERIOS DE SELECCIÓN: Ensayos controlados aleatorizados (ECA) que compararan: 1) intervenciones farmacológicas con placebo; 2) estimulación cerebral no invasiva con estimulación simulada o atención habitual; 3) terapia psicológica con atención habitual o control de atención; 4) intervención farmacológica y terapia psicológica con intervención farmacológica y atención habitual o control de atención; 5) intervención farmacológica y estimulación cerebral no invasiva con intervención farmacológica y estimulación simulada o atención habitual; 6) estimulación cerebral no invasiva y terapia psicológica versus estimulación cerebral simulada o atención habitual y terapia psicológica; 7) intervención farmacológica y terapia psicológica con placebo y terapia psicológica; 8) intervención farmacológica y estimulación cerebral no invasiva con placebo y estimulación cerebral no invasiva; y 9) estimulación cerebral no invasiva y terapia psicológica versus estimulación cerebral no invasiva y atención habitual o control de atención, con la intención de tratar la depresión después del accidente cerebrovascular. OBTENCIÓN Y ANÁLISIS DE LOS DATOS: Dos autores de la revisión, de forma independiente, seleccionaron los estudios, evaluaron el riesgo de sesgo y extrajeron los datos de los estudios incluidos. Se calculó la diferencia de medias (DM) o la diferencia de medias estandarizada (DME) para los datos continuos, y la razón de riesgos (RR) para los datos dicotómicos, con intervalos de confianza (IC) del 95%. La heterogeneidad se evaluó mediante la estadística I² y la certeza de la evidencia según GRADE.
Resultados principales: Se incluyeron 65 ensayos (72 comparaciones) con 5831 participantes. Se dispuso de datos para: 1) 20 comparaciones; 2) nueve comparaciones; 3) 25 comparaciones; 4) tres comparaciones; 5) 14 comparaciones; y 6) una comparación. No se encontraron ensayos para las comparaciones 7 a 9. Comparación 1: Intervenciones farmacológicas Evidencia de certeza muy baja de ocho ensayos indica que las intervenciones farmacológicas disminuyeron el número de personas que cumplían los criterios del estudio para la depresión (RR 0,70; IC del 95%: 0,55 a 0,88; p = 0,002; ocho ECA; 1025 participantes) al final del tratamiento y evidencia de certeza muy baja de seis ensayos indica que las intervenciones farmacológicas disminuyeron el número de personas con respuesta inadecuada al tratamiento (RR 0,47; IC del 95%: 0,32 a 0,70; p = 0,0002; seis ECA; 511 participantes) en comparación con placebo. Se observaron más eventos adversos relacionados con el sistema nervioso central (SNC) (RR 1,55; IC del 95%: 1,12 a 2,15; p = 0,008; cinco ECA; 488 participantes; evidencia de certeza muy baja) y el sistema gastrointestinal (RR 1,62; IC del 95%: 1,19 a 2,19; p = 0,002; cuatro ECA; 473 participantes; evidencia de certeza muy baja) en el grupo de intervención farmacológica que en el grupo placebo. Comparación 2: Estimulación cerebral no invasiva Evidencia de certeza muy baja de dos ensayos muestra que la estimulación cerebral no invasiva tuvo poco o ningún efecto sobre el número de personas que cumplían los criterios del estudio para la depresión (RR 0,67; IC del 95%: 0,39 a 1,14; p = 0,14; dos ECA; 130 participantes) y el número de personas con respuesta inadecuada al tratamiento (RR 0,84; IC del 95%: 0,52 a 1,37; p = 0,49; dos ECA; 130 participantes) en comparación con la estimulación simulada. La estimulación cerebral no invasiva no provocó muertes. Comparación 3: Terapia psicológica Evidencia de certeza muy baja de seis ensayos indica que la terapia psicológica disminuyó el número de personas que cumplían los criterios del estudio para la depresión al final del tratamiento (RR 0,77; IC del 95%: 0,62 a 0,95; p = 0,01; 521 participantes) en comparación con atención habitual/control de atención. Ningún ensayo de terapia psicológica informó sobre el desenlace respuesta inadecuada al tratamiento. No se encontraron diferencias en el número de muertes o eventos adversos en el grupo de terapia psicológica en comparación con el grupo de control de atención/atención habitual. Comparación 4: Intervenciones farmacológicas con terapia psicológica Ningún ensayo de esta combinación informó sobre los desenlaces principales. El tratamiento combinado no provocó muertes. Comparación 5: Intervenciones farmacológicas con estimulación cerebral no invasiva La estimulación cerebral no invasiva con intervención farmacológica redujo el número de personas que cumplían los criterios del estudio para la depresión al final del tratamiento (RR 0,77; IC del 95%: 0,64 a 0,91; p = 0,002; tres ECA; 392 participantes; evidencia de certeza baja), pero no el número de personas con respuesta inadecuada al tratamiento (RR 0,95; IC del 95%: 0,69 a 1,30; p = 0,75; tres ECA; 392 participantes; evidencia de certeza muy baja) en comparación con el tratamiento farmacológico solo. Evidencia de certeza muy baja de cinco ensayos no indica diferencias en las muertes entre este tratamiento combinado (RR 1,06; IC del 95%: 0,27 a 4,16; p = 0,93; 487 participantes) en comparación con la intervención de tratamiento farmacológico y la estimulación simulada o la atención habitual. Comparación 6: Estimulación cerebral no invasiva con terapia psicológica Ningún ensayo de esta combinación informó sobre los desenlaces principales.
Conclusiones de los autores: Evidencia de certeza muy baja indica que los tratamientos farmacológicos, las terapias psicológicas y los tratamientos combinados pueden reducir la prevalencia de la depresión, mientras que la estimulación cerebral no invasiva tuvo poco o ningún efecto sobre la prevalencia de la depresión. Las intervenciones farmacológicas se asociaron con eventos adversos relacionados con el SNC y el sistema gastrointestinal. Se necesitan más estudios de investigación antes de poder hacer recomendaciones sobre el uso habitual de dichos tratamientos.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
SA: none known. KC: none known. C‐FH: none known. HL: none known. AH: none known. MH: none known.
Figures



















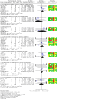

























































Update of
-
Pharmacological, psychological, and non-invasive brain stimulation interventions for treating depression after stroke.Cochrane Database Syst Rev. 2020 Jan 28;1(1):CD003437. doi: 10.1002/14651858.CD003437.pub4. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Jul 5;7:CD003437. doi: 10.1002/14651858.CD003437.pub5. PMID: 31989584 Free PMC article. Updated.
References
References to studies included in this review
Alexopoulos 2012 {published data only}
-
- Kiosses DN, Alexopoulos GS, Wilkins V. Ecosystem focused therapy for treating older depressed stroke survivors. http://www.strokecenter.org/trials/clinicalstudies/ecosystem-focused-the... (first received 22 July 2009).
-
- NCT00944762. Ecosystem focused therapy for treating older depressed stroke survivors. http://ClinicalTrials.gov/show/NCT00944762 (first received 23 July 2009).
Andersen 1994 {published data only}
-
- Andersen G, Vestergaard K, Lauritzen L. Effective treatment of post-stroke depression with the selective serotonin reuptake inhibitor, citalopram. Journal of Neurology 1994;241 Suppl 1:S42. - PubMed
-
- Andersen G, Vestergaard K, Lauritzen L. Effective treatment of poststroke depression with the selective serotonin reuptake inhibitor citalopram. Stroke 1994;25(6):1099-104. - PubMed
-
- Andersen G, Vestergaard K, Lauritzen L. Post-stroke depression treated with citalopram - a selective serotonin reuptake inhibitor. Canadian Journal of Neurological Sciences 1993;20(Suppl 4):S115.
-
- Andersen G, Vestergaard K, Lauritzen L. Post-stroke depression treated with citalopram a selective serotonin reuptake inhibitor. In: Proceedings of the 7th Scandinavian Meeting on Cerebrovascular Disease. Jyvaskyla, Finland, 14-17 August 1993:54.
-
- Andersen G, Vestergaard K, Lauritzen L. Post-stroke depression treated with citalopram. Acta Neurologica Scandinavica 1994;89 Suppl 155:20.
Cao 2009a {published data only}
-
- Cao WW, Yu JM, Sun SY, Sun YB, Luan L, Cai XJ, et al. Group psychotherapy in treatment of post stroke depression [团体心理治疗在脑卒中后抑郁治疗中的应用]. Chinese Mental Health Journal 2009;23(2):100-4.
Cao 2009b {published data only}
-
- Cao WW, Yu JM, Sun SY, Sun YB, Luan L, Cai XJ, et al. Group psychotherapy in treatment of post stroke depression [团体心理治疗在脑卒中后抑郁治疗中的应用]. Chinese Mental Health Journal 2009;23(2):100-4.
Chen 2005a {published data only}
-
- Chen Y-P, Mei Y-W, Sun S-G, Bao M, Yu S-C. Evaluation of frequency repetitive transcranial magnetic stimulation for post-stroke depression and neurologic impairment. Zhongguo Linchuang Kangfu 2005;9:18-9.
Cullen 2018 {published data only}
-
- Cullen B, Pownall J, Cummings J, Baylan S, Broomfield N, Haig C, et al. Positive PsychoTherapy in ABI Rehab (PoPsTAR): a pilot randomised controlled trial. Neuropsychological Rehabilitation 2018;28(1):17-33. - PubMed
Du 2005 {published data only}
-
- Du DQ, Wu YB. Living ability and cognitive function ameliorated by low frequency repetitive transcranial magnetic stimulation in patients with post-stroke depression: comparison with drug plus psychological treatment. Zhongguo Linchuang Kangfu 2005;9(16):22-3.
Fan 2010 {published data only}
-
- Fan J, He H. Effect of psychological nursing on rehabilitation of patients with post-stroke depression. Journal of Clinical and Experimental Medicine 2010;29(9):1335–8.
Fan 2014 {published data only}
-
- Fan X. Duloxetine combined with repetitive transcranial magnetic stimulation in the treatment of post stroke depression effect. Chinese Journal of Practical Nervous Disease 2014;1:102-3.
Fang 2017 {published data only}
Fruehwald 2003 {published data only}
-
- Fruehwald S, Gatterbauer E, Rehak P, Baumhackl U. Early fluoxetine treatment of post-stroke depression: a three months double-blind placebo-controlled study with an open-label long-term follow up. Journal of Neurology 2003;250(3):347-51. - PubMed
Gao 2017a {published data only}
-
- Gao J, Lin M, Zhao J, Bi S, Ni Z, Shang X. Different interventions for post-ischaemic stroke depression in different time periods: a single-blind randomized controlled trial with stratification by time after stroke. Clinical Rehabilitation 2017;31(1):71-81. - PubMed
Gao 2017b {published data only}
-
- Gao J, Lin M, Zhao J, Bi S, Ni Z, Shang X. Different interventions for post-ischaemic stroke depression in different time periods: a single-blind randomized controlled trial with stratification by time after stroke. Clinical Rehabilitation 2017;31(1):71-81. - PubMed
Gu 2016 {published data only}
-
- Gu SY, Chang MC. The effects of 10-Hz repetitive transcranial magnetic stimulation on depression in chronic stroke patients. Brain Stimulation 2016;10:270-4. - PubMed
Hoffmann 2015 {published and unpublished data}
-
- ACTRN12609000741280. Evaluation of brief interventions for enhancing early emotional adjustment following stroke: a pilot randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=308355&... (first received 25 August 2009).
-
- Hoffmann T, Ownsworth T, Eames S, Shum D. Evaluation of brief interventions for enhancing early emotional adjustment following stroke: a pilot randomised controlled trial. Topics in Stroke Rehabilitation 2015;22(2):117-26. - PubMed
-
- Ownsworth T, Hoffman T, Stemm B, Evans E, Howlett J, Shum D. Early cognitive appraisals, benefit finding and emotional status after stroke: pre-intervention associations and preliminary intervention findings. Brain Impairment 2011;12:66.
Hordacre 2021 {published and unpublished data}
-
- Hordacre B, Comacchio K, Williams L, Hillier S. Repetitive transcranial magnetic stimulation for post-stroke depression: a randomised trial with neurophysiological insight. Journal of Neurology 2021;268:1474-84. - PubMed
Huang 2002 {published data only}
-
- Huang, XH. The clinical correlation study and the effect of fluoxetine intervention on poststroke depression. Chinese Journal of Clinical Rehabilitation 2002;6(15):2296-7.
Jiang 2001a {published data only}
-
- Jiang B, Lu W, Song X-W, Tan L-M, Hu Z-P. The effect of poststroke depression interventions on the recovery of neurological function. Modern Rehabilitation 2001;5(3):29-30.
Jiang 2001b {published data only}
-
- Jiang B, Lu W, Song X-W, Tan L-M, Hu Z-P. The effect of poststroke depression interventions on the recovery of neurological function. Modern Rehabilitation 2001;5(3):29-30.
Jiang 2014a {published data only}
-
- Jiang X. Effect of transcranial magnetic stimulation combined with sertraline on neurological deficits in patients with acute cerebral infarction. Chinese Journal of Practical Nervous Disease 17;21:81-3.
Jiang 2014b {published data only}
-
- Jiang X. Effect of transcranial magnetic stimulation combined with sertraline on neurological deficits in patients with acute cerebral infarction. Chinese Journal of Practical Nervous Disease 2014;17(21):81-3.
Jin 2013 {published data only}
-
- Jin H. Effect of repetitive transcranial magnetic stimulation on post stroke depression. Chinese Journal of Rehabilitation Medicine 2013;28(1):58-60.
Kerr 2018 {published data only}
-
- ACTRN12617000245392. Effects of early motivational interviewing on post-stroke depressive symptoms: a pilot randomised controlled trial of the Good Mood Intervention program. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372347 (first received 13 February 2017). - PubMed
-
- Kerr D, Mackey E, Wijeratne T, McCann T, Rhodes L. Early motivational interviewing on post-stroke depressive symptoms: the good mood intervention program-pilot RCT. International Journal of Stroke 2015;10 Suppl 3:1-84. - PubMed
-
- Kerr D, Mackey E, Wijeratne T, McCann T. Nursing early motivational interviewing on poststroke depressive symptoms: pilot randomized controlled trial of the good mood intervention program. International Journal of Stroke 2014;19:245. - PubMed
-
- Kerr D, McCann T, Mackey E, Wijeratne T. Effects of early motivational interviewing on post-stroke depressive symptoms: pilot randomised study of the Good Mood Intervention program. International Journal of Nursing Practice 2018;24:1-8. - PubMed
Kirkness 2017a {published data only}
-
- Byun, Eeeseung, Becker, Kyra J, Kohen, Ruth, Kirkness, Catherine J, Mitchell, Pamela H. Brief psychosocial intervention to address poststroke depression may also benefit fatigue and sleep-wake disturbance. Rehabilitation Nursing 2021;46(4):222-231. - PubMed
Kirkness 2017b {published data only}
-
- Byun, Eeeseung, Becker, Kyra J, Kohen, Ruth, Kirkness, Catherine J, Mitchell, Pamela H. Brief psychosocial intervention to address poststroke depression may also benefit fatigue and sleep-wake disturbance. Rehabilitation Nursing 2021;46(6):222-231. - PubMed
Kong 2007 {published data only}
-
- Kong Y, Dong WL, Liu CF. Fluoxetine for poststroke depression: a randomized placebo controlled clinical trial. Neural Regeneration Research 2007;2:162-5.
Lai 2006a {published data only}
-
- Lai J, Zeng G. The effect of using paroxetine to treat post stroke depression. Journal of Guangdong Medical College 2006;24(6):585-6.
Li 2008 {published data only}
-
- Li L, Wang S, Ge H, Chen J, Yue S, Yu M. The beneficial effects of the herbal medicine Free and Easy Wanderer Plus (FEWP) and fluoxetine on post-stroke depression. Journal of Alternative & Complementary Medicine 2008;14(7):841-6. - PubMed
Li 2009 {published data only}
-
- Li X, Lai Z. Psychological care of patients with post-stroke depression. Journal of Clinical and Experimental Medicine 2009;8:4-5.
Li 2013 {published data only}
-
- Li N, Han Y. A study of efficacy mirtazapine merge rTMS to treatment of post-stroke depression. Chinese Journal of Trauma and Disability Medicine 2013;21(6):48-50.
Li 2014 {published data only}
-
- Li L. Fluoxetine capsules combined with repetitive transcranial magnetic stimulation on depression after stroke treatment. Chinese Journal of Practical Nervous Disease 2014;17(20):105-6.
Li 2019a {published data only}
-
- Yanfang L, Yang W. Effect of early psychological nursing intervention on neuropsychological improvement of patients with poststroke depression. Shanxi Medical Journal 2019;48:2101-3.
Liang 2015 {published data only}
-
- Liang S, Wu Z. Effect of psychological nursing intervention on mental health and quality of life for stroke patients with depression and anxiety. Chinese Journal of Nod Nursing 2015;16:1912-4.
Lincoln 2003 {published data only}
-
- Flannaghan T. Cognitive behavioural psychotherapy for the treatment of depression after stroke. Unpublished 2000.
-
- Lincoln N. Pilot evaluation of cognitive behavioural treatment of depression after stroke. National Research Register 1996. - PubMed
-
- Lincoln NB, Flannaghan T. Cognitive behavioral psychotherapy for depression following stroke: a randomized controlled trial. Stroke 2003;34:111-5. - PubMed
-
- Thomas SA, Lincoln NB. Factors relating to depression after stroke. British Journal of Clinical Psychology 2006;45:49-61. - PubMed
Lipsey 1984 {published data only}
-
- Kimura M, Robinson RG, Kosier JT. Treatment of cognitive impairment after poststroke depression: a double-blind treatment trial. Stroke 2000;31:1482-6. - PubMed
-
- Kimura M, Tateno A, Robinson RG. Treatment of poststroke generalized anxiety disorder comorbid with poststroke depression: merged analysis of nortriptyline trials. American Journal of Geriatric Psychiatry 2003;11(3):320-7. - PubMed
-
- Lipsey JR, Robinson RG, Pearlson GD, Rao K, Price TR. Nortriptyline treatment of post-stroke depression: a double-blind study. Lancet 1984;1(8372):297-300. - PubMed
-
- Lipsey JR, Robinson RG. Nortriptyline for post-stroke depression. Lancet 1984;1(8380):803. - PubMed
Liu 2015 {published data only}
-
- Liu X. The effect of repetitive transcranial magnetic stimulation on the treatment of post-stroke depression. Chinese Journal of Gerontology 2015;1:5621-2.
Liu 2020 {published data only}
-
- Liu W, Ding W. Study on the efficacy and mechanism of paroxetine hydrochloride combined with repetitive transcranial magnetic stimulation in the treatment of post-stroke depression. International Journal of Clinical and Experimental Medicine 2020;13(10):7881-8.
Lu 2016 {published data only}
-
- Lu Q, Zhang H-P, Yang S-P, Zhu Z-F. Study of low frequency repetitive transcranial magnetic stimulation in the treatment of post-stroke depression. Hainan Medical Journal 2016;27(12):1963-4.
Lu 2018 {published data only}
-
- Lu X, Li M, Zhao Y. Clinical observation on the application of psychological intervention and humanistic care nursing in patients with post-stroke depression. Journal of Qiqihar University Medicine 2018;39:2066-7.
Lu 2020 {published data only}
Meng 2015 {published data only}
-
- Meng Y. Clinical study of transcranial magnetic therapy in the treatment of depression after cerebral infarction. Chinese Journal of Trauma and Disability Medicine 2015;23(5):141-2.
Mitchell 2002 {published data only}
-
- Barer D. A brief psychosocial-behavioral intervention reduced depression after stroke more than usual care: commentary. Annals of Internal Medicine 2010;152:JC3-10. - PubMed
-
- Mitchell PH, Becker KJ, Buzaitis A, Cain KC, Johnson V, Kohen R, et al. Factors associated with treatment response to combined psychosocial and antidepressant treatment of post-stroke depression (PSD). Stroke 2010;41:e235-6.
-
- Mitchell PH, Teri L, Veith R, Buzaitis A, Tirschwell D, Becker K, et al. Living well with stroke: design and methods for a randomized controlled trial of a psychosocial behavioral intervention for poststroke depression. Journal of Stroke and Cerebrovascular Diseases 2008;17(3):109-15. - PMC - PubMed
Murray 2002 {published data only}
-
- Murray V, Von Arbin M, Asberg M, Bartfai A, Berggren A, Landtblom A, et al. Double-blind placebo comparison of sertraline and placebo in stroke patients with depression. Unpublished 2003.
-
- Murray V, Von Arbin M, Bartfai A, Berggren A, Landtblom A, Lundmark J, et al. Double-blind comparison of sertraline and placebo in stroke patients with minor depression and less severe major depression. Journal of Clinical Psychiatry 2005;66(6):708-16. - PubMed
-
- Murray V, Von Arbin M, Varelius R, Olsson JE, Terent A, Samuelsson M, et al. Sertraline in poststroke depression: a controlled study. Stroke 2002;33(1):P292.
Ohtomo 1991 {published data only}
-
- Kumar V. Post-stroke depression and treatment strategies including aniracetam. International Journal of Geriatric Psychopharmacology 1999;2:40-6.
-
- Ohtomo E, Hirai S, Terashi A, Hasegawa K, Tazaki Y, Araki G, et al. Clinical evaluation of aniracetam on psychiatric symptoms related to cerebrovascular disease. Journal of Clinical Experimental Medicine 1991;156:143-87.
Ponzio 2001 {published data only}
-
- An 8-week, double-blind, placebo controlled, parallel group study to assess the efficacy and tolerability of paroxetine in patients suffering from depression following stroke. http://www.ctr.gsk.co.uk/Summary/Paroxetine/III_PAR_625.pdf;(par 625).
-
- Ponzio F, Marini G, Riva E. The efficacy of paroxetine in some kinds of "critical" patients. European Neuropsychopharmacology 2001;11 Suppl 2:S49-S50 Abstract P.1.29.
Rampello 2005 {published data only}
-
- Rampello L, Alvano A, Chiechio S, Raffaele R, Vecchio I, Malaguarnera M. An evaluation of efficacy and safety of reboxetine in elderly patients affected by "retarded" post-stroke depression: a random, placebo-controlled study. Archives of Gerontology and Geriatrics 2005;40:275-85. - PubMed
Reding 1986 {published data only}
-
- Reding MJ, Orto LA, Winter SW, Fortuna IM, Di Ponte P, McDowell FH. Antidepressant therapy after stroke: a double-blind trial. Archives of Neurology 1986;43(8):763-5. - PubMed
Robinson 2008a {published data only}
-
- Robinson RG, Jorge RE, Clarence-Smith K, Starkstein S. Double-blind treatment of apathy in patients with poststroke depression using nefiracetam. Journal of Neuropsychiatry and Clinical Neurosciences 2009;21:144-51. - PubMed
-
- Robinson RG, Jorge RE, Clarence-Smith K. Double-blind randomized treatment of poststroke depression using nefiracetam. Journal of Neuropsychiatry and Clinical Neurosciences 2008;20(2):178-84. - PubMed
Robinson 2008b {published data only}
-
- Robinson RG, Jorge RE, Clarence-Smith K, Starkstein S. Double-blind treatment of apathy in patients with poststroke depression using nefiracetam. Journal of Neuropsychiatry and Clinical Neurosciences 2009;21:144-51. - PubMed
-
- Robinson RG, Jorge RE, Clarence-Smith K. Double-blind randomized treatment of poststroke depression using nefiracetam. Journal of Neuropsychiatry and Clinical Neurosciences 2008;20(2):178-84. - PubMed
Sun 2013 {published data only}
-
- Sun H. The efficacy of repetitive transcranial magnetic stimulation in the treatment of post-stroke depression. Chinese Journal of Integrative Medicine on Cardio-Cerebrovascular Disease 2013;11(3):321-2.
Tao 2008 {published data only}
-
- Tao M, Yang X. Effect of psychological nursing on depressive symptoms and functional recovery of patients with stroke depression. Chinese Journal of Practical Nervous Diseases 2008;11:154.
Terachinda 2021 {published data only}
-
- Terachinda P, Tantanatip A, Piravej K, Tangwongchai S, Srioun S, Sillapachar T, et al. High frequency repetitive transcranial magnetic stimulation versus setraline in treatment of poststroke depression: a pilot study. Chulalongkorn Medical Journal 2021;65(1):71-9.
Thomas 2007 {published data only}
-
- ISRCTN56078830. Communication And Low Mood (CALM) study. https://www.isrctn.com/ISRCTN56078830 (first received 24 June 2010).
-
- ISRCTN63855912. Psychological treatment for depression in aphasic stroke patients: a randomised controlled trial. https://doi.org/10.1186/ISRCTN63855912 (first received 29 September 2006).
-
- N0192165295. CALM: communication and low mood. National Research Register (first received 23 September 2009).
-
- Thomas SA, Lincoln NB, Walker MF, Macniven J, Haworth H. Communication and low mood (CALM) study: a randomised controlled trial evaluating behaviour therapy for low mood in people with aphasia after stroke. International Journal of Stroke 2011;6 Suppl 2:27-8.
Thomas 2016 {published data only}
-
- ISRCTN12715175. Behavioural activation therapy for depression after stroke. https://doi.org/10.1186/ISRCTN12715175 (first received 10 December 2014).
-
- Thomas SA, Coates E, das Nair R, Lincoln NB, Cooper C, Palmer R, et al. BEhavioural Activation therapy for Depression after Stroke (BEADS): a study protocol for a feasibility randomised controlled pilot trial of a psychological intervention for post-stroke depression. Pilot and Feasibility Studies 2016;2(45):1-12. - PMC - PubMed
-
- Thomas SA, Drummond AER, Lincoln NB, Palmer RL, das Nair R, Latimer NR, Hackney GL, Mandefield L, Walters SJ, Hatton RD, Cooper CL, Chater TF, England TJ, Callaghan P, Coates E, Sutherland KE, Eshtan SJ, Topcu G. Behavioural activation therapy for post-stroke depression: the BEADS feasibility RCT. Health Technology Assessment 2019;23(47):1-206. - PMC - PubMed
Tian 2010 {published data only}
-
- Tian Y, Ren A. Observation on the effect of psychological care on the patients anxiety and depression disorders after stroke. Journal Qilu Nursing 2010;16:6-7.
Towle 1989 {published data only}
-
- Towle D, Lincoln NB, Mayfield LM. Evaluation of social work on depression after stroke. Clinical Rehabilitation 1989;3(2):89-96.
-
- Towle D, Mayfield L, Lincoln M. Depression after stroke. Clinical Rehabilitation 1988;2:256.
Valiengo 2017 {published data only}
-
- NCT01525524. Treatment of major depressive disorder post stroke with transcranial direct current stimulation. https://clinicaltrials.gov/ct2/show/NCT01525524 (first received 3 February 2012).
-
- Valiengo LCL, Goulart AC, Oliveira JF, Benseñor IM, Lotufo PA, Brunoni AR. Transcranial direct current stimulation for the treatment of post-stroke depression: results from a randomised, sham-controlled, double-blinded trial. Journal of Neurology, Neurosurgery and Psychiatry 2017;88:170-5. - PubMed
Wang 2004a {published data only}
-
- Wang X, Song J, Mu J. The effect of psychotherapy on depression and cognitive function of patients with cerebral stroke. Chinese Mental Health Journal 2004;18:778-81.
Wang 2005 {published data only}
-
- Chen Y, Guo JJ, Zhan S, Paul NC. Treatment effects of antidepressants in patients with post-stroke depression: a meta-analysis. Annals of Pharmacotherapy 2006;40:2115-22. - PubMed
-
- Wang ZM, Wang P, You LL. Study of effects of fluoxetine in patients with post-stroke depression, a random placebo-controlled study. Chinese Journal of Practical Nervous Diseases 2005;8:80-1.
Wang 2005a {published data only}
-
- He Y, Wang X. A clinical study of paroxetine joint psychotherapy in treating poststroke depression with anxiety. Chinese Journal of Practical Nervous Diseases 2006;9(1):34-5.
-
- Wang X, He Y, Xiao CL. A clinical trial of paroxetine and psychotherapy in patients with poststroke depression and anxiety. Chinese Mental Health Journal 2005;19:564-6.
Wang 2019 {published data only}
-
- Wang Z, Cai X, Xin H. Effect of psychological counseling nursing on training mentality and quality of life of patients with post-stroke depression. Mod Diagn Treat 2019;30:2528-30.
Watkins 2007 {published and unpublished data}
-
- Deans CF, Jack CIA. Evaluation of motivational interviewing early after acute stroke: a randomized controlled trial. Clinical Rehabilitation 2006;20:731-6.
-
- Sutton C, Dickinson H, Leathley M, Hills K, Auton M, Lightbody E, et al. Motivational interviewing: altering outcome after stroke. In: 12th European Stroke Conference. Valencia, Spain, 2003 May 21-24:103.
-
- Watkins CL, Auton MF, Deans CF, Dickinson HA, Jack CIA, Lightbody CE, et al. Motivational interviewing early after acute stroke: a randomized, controlled trial. Stroke 2007;38:1004-9. - PubMed
-
- Watkins CL, Wathan JV, Leathley MJ, Auton MF, Deans CF, Dickinson HA, et al. The 12-month effects of early motivational interviewing after acute stroke: a randomized controlled trial. Stroke 2011;42:1956-61. - PubMed
Wei 2021 {published data only}
Wiart 2000 {published data only}
-
- Wiart L, Gassies TD, France B, Petit H, Debelleix D. A double-blind, placebo controlled trial to study the efficacy and tolerance of fluoxetine in the treatment of early post stroke depression. In: Proceedings of the 152nd Annual Meeting of the American Psychiatric Association. USA, Washington: American Psychiatric Association, 15-20 May 1999.
-
- Wiart L, Petit H, Joseph PA, Mazaux JM, Barat M. Fluoxetine in early poststroke depression: a double-blind placebo-controlled study. Stroke 2000;31(8):1829-32. - PubMed
Wu 2019 {published data only}
-
- Wu J, Li M, Liu J. Therapeutic effect of transcranial magnetic stimulation combined with cognitive therapy on post-stroke depression. International Journal of Psychiatry and Neurology 2019;8(2):13-8.
Yang 2002 {published data only}
-
- Yang J, Zhao Y, Bai S. Controlled study on antidepressant treatment of patients with post-stroke depression. Chinese Journal of Psychology 2002;16(12):871-2.
Yang 2013 {published data only}
-
- Yang M. A comparative study of high frequency repetitive transcranial magnetic stimulation in the treatment of post stroke depression. Stroke Nervous Disorders 2013;20(5):303-5.
Yang 2014a {published data only}
-
- Yang L, Liu Y, Liu, L, Qi, X, Shi W, Lu C, et al. The curative effect of different frequency repetitive transcranial magnetic stimulation on patients with depression after stroke. Chinese Journal of Practical Nervous Diseases 2014;17(22):18-20.
Yang 2014b {published data only}
-
- Yang L, Liu Y, Liu, L, Qi, X, Shi W, Lu C, et al. The curative effect of different frequency repetitive transcranial magnetic stimulation on patients with depression after stroke. Chinese Journal of Practical Nervous Diseases 2014;17(22):18-20.
Zhang 2013 {published data only}
-
- Zhang Z, Mu J, Geng G-H, Li Q, Song J-G. Effects of repetitive transcranial magnetic stimulation on depression and cognition in the treatment of post-stroke depression. Chinese Journal of Physical Medical Rehabilitation 2013;35(3):197-200.
Zhao 2004 {published data only}
-
- Zhao H-W, Zhou C-X, Su X-L, Xiao X-C, Guo Y. Effect of mental intervention on post-stroke depression and rehabilitation of neurological function. Chinese Journal of Clinical Rehabilitation 2004;8(13):2408-9.
Zheng 2016 {published data only}
-
- Zheng F. Clinical observation of ultra-low frequency transcranial magnetic stimulation in the treatment of post stroke depression. Today Nurse 2016;6:116-7.
References to studies excluded from this review
Aben 2014 {published data only}
-
- Aben L, Heijenbrok-Kal MH, Ponds RWHM, Busschbach JJV, Ribbers GM. Long-lasting effects of a new memory self-efficacy training for stroke patients: a randomized controlled trial. Neurorehabilitation and Neural Repair 2014;28(3):199-206. - PubMed
-
- Aben L, Heijenbrok-Kal MH, Loon EMP, Groet E, Ponds RWHM, Busschbach JJV, et al. Training memory self-efficacy in the chronic stage after stroke: a randomized controlled trial. Neurorehabilitation and Neural Repair 2013;27(2):110-7. - PubMed
ACTRN12615000840583 {published data only}
-
- ACTRN12615000840583. The efficacy of problem solving therapy for treating depression in younger (18-65) stroke patients compared to a social control group. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369019 (first received 13 August 2015).
ACTRN12620001174976 {published data only}
-
- ACTRN12620001174976. Modified and tailored cognitive behavioural therapy to treat depression for stroke survivors with aphasia. https://www.australianclinicaltrials.gov.au/anzctr/trial/ACTRN1262000117... (first received 18 January 2020).
Agnoli 1985 {published data only}
-
- Agnoli A, Fioravanti M, Lechner H. Efficacy of CDP-Choline in chronic cerebral vascular diseases (CCVD). In: Appia V, Kennedy EP, Nilsson BI, Galletti P, editors(s). Novel Biochemical, Pharmacological and Clinical Aspects of Cytidinediphosphocholine. New York: Elsevier, 1985:305-15.
Bai 2017 {published data only}
-
- Bai B, Yan Z, Hao Y, Zhang Z, Li G, Dekker J, et al. A randomised controlled multimodal intervention trial in patients with ischaemic stroke in Shandong, China: design and rationale. Lancet 2017;390(1):13.
Bai 2019 {published data only}
Beauchamp 2020 {published data only}
-
- Beauchamp JE, Chaoul A, Cron S, Montiel TC, Payen S, Prossin A, et al. Meditation for poststroke depression: a pilot randomized controlled trial. Stroke 2020;51:2.
Bramanti 1989 {published data only}
-
- Bramanti P, Ricci RM, Di Bella P, De Luca GP, Sessa E, Di Leo M, et al. Neuropsychological and clinical evaluation after administration of TRH-T in cerebrovascular pathology [Valutazioni neuropsicologiche e cliniche dopo somminitrazione di TRH-T neila patologia cerebrovascolare]. Rassegna di Medicina Interna 1989;X(4):157-61.
Casella 1960 {published data only}
-
- Casella C, Sokolow J. A study to determine the energizing effects of iproniazid (marsilid) on a group of hemiplegics. Archives of Physical Medicine and Rehabilitation 1960;41:381-5. - PubMed
Chalmers 2019 {published data only}
-
- Chalmers C, Leathem J, Bennett S, McNaughton H, Mahawish K. The efficacy of problem solving therapy to reduce post stroke emotional distress in younger (18-65) stroke survivors. Disability Rehabilitation 2019;41(7):753-62. - PubMed
Chang 2011 {published data only}
-
- Chang K, Zhang H, Xia Y, Chen C. Testing the effectiveness of knowledge and behavior therapy in patients of hemiplegic stroke. Topics in Stroke Rehabilitation 2011;18(5):525-35. - PubMed
Chen 2019 {published data only}
-
- Chen L, Wang F, Lv L, Zhang Y, Shen X. The efficacy of a patient-centered self-management empowerment intervention program (PCSMEI) for first-time stroke survivors: a randomized controlled trial. Stroke 2019;50 Suppl 1:AWP186.
Cheng 2016 {published data only}
-
- Cheng HY. The effect of a psychoeducational intervention on stroke family caregivers' outcomes and stroke survivors' utilisation of health and social services. Dissertation Abstracts International: Section B: The Sciences and Engineering 2016;76.
Cheng 2020 {published data only}
-
- Cheng C, Fan W, Liu C, Liu Y, Liu X. Reminiscence therapy based care program relieves post-stroke cognitive impairment, anxiety, and depression in acute ischemic stroke patients: a randomized, controlled study. Irish Journal of Medical Science 2021;190(1):345-55. - PubMed
ChiCTR1800016101 {published data only}
-
- ChiCTR1800016101. The effectiveness and cost-effectiveness of a virtual multidisciplinary stroke care clinic for community-dwelling stroke survivors and caregivers: a randomised controlled trial. https://www.chictr.org.cn/showprojen.aspx?proj=27387 (first received 5 November 2018).
ChiCTR1800017752 {published data only}
-
- ChiCTR1800017752. Exploring clinical efficacy evaluation of psychological and physical rehabilitation on post-stroke patients: a Zelen's design study. https://www.chictr.org.cn/showproj.aspx?proj=26300 (first received 12 August 2018).
ChiCTR1800019366 {published data only}
-
- ChiCTR1800019366. Escitalopram in 8-week open-label treatment of post-stroke depression: the efficacy, safety and the effect on regional brain function. https://www.chictr.org.cn/showproj.aspx?proj=32490 (first received 7 November 2018).
ChiCTR1900021168 {published data only}
-
- ChiCTR1900021168. Resting state EEG predicts the efficacy of rTMS treatment regimen for post-stroke depression. https://www.chictr.org.cn/showproj.aspx?proj=35725 (first received 31 January 2019).
ChiCTR1900026358 {published data only}
-
- ChiCTR1900026358. Mechanism of Shugan Jieyu Capsule for improving cognitive function in altered dynamics of brain of post-stroke depression patients. https://www.chictr.org.cn/showprojen.aspx?proj=43924 (first received 10 February 2019).
ChiCTR2000029450 {published data only}
-
- ChiCTR2000029450. The efficacy and safety of bright light therapy combined with escitalopram oxalate in the treatment of sleep disorders associated with post-stroke depression. https://www.chictr.org.cn/showproj.aspx?proj=40152 (first received 2 February 2020).
ChiCTR2000029554 {published data only}
-
- ChiCTR2000029554. Traditional Chinese medicine (TCM) collaborative care for patients with poststroke depression: a randomized controlled trial. https://www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR2000029554 (first received 2 April 2020).
ChiCTR2000035588 {published data only}
-
- ChiCTR2000035588. Clinical efficacy of “Conduct Qi with Mindwill” exercise in intervening early post-stroke depression and characteristics of electroencephalogram signals. https://www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR2000035588 (first received 14 August 2020).
ChiCTR2000036944 {published data only}
-
- ChiCTR2000036944. A randomized controlled clinical study of Nerve Fuyuan Decoction in the treatment of post-ischemic depression after stroke. https://www.chictr.org.cn/historyversionpub.aspx?regno=ChiCTR2000036944 (first received 25 August 2020).
ChiCTR2000039143 {published data only}
-
- ChiCTR2000039143. Study on quantitative diagnosis model of TCM syndromes of post-stroke depression based on combination of disease and syndrome. https://www.chictr.org.cn/historyversionpuben.aspx?regno=ChiCTR2000039143 (first received 1 January 2020). - PMC - PubMed
ChiCTR2000039459 {published data only}
-
- ChiCTR2000039459. The efficacy and mechanism of repetitive transcranial magnetic stimulation in the treatment of post-stroke depression. https://www.chictr.org.cn/showproj.aspx?proj=62973 (first received 30 January 2021).
ChiCTR2100042684 {published data only}
-
- ChiCTR2100042684. Effectiveness of a family-focused dyadic psychoeducational intervention (FDPEI) for stroke survivors and family caregivers on functional and psychosocial well-being: a randomised controlled trial. https://www.chictr.org.cn/showproj.aspx?proj=65230 (first received 26 January 2021).
Choi‐Kwon 2006 {published data only}
-
- Choi-Kwon S, Choi J, Kwon SU, Kang D, Kim JS. Fluoxetine is not effective in the treatment of poststroke fatigue: a double-blind, placebo controlled study. Cerebrovascular Diseases 2007;23:103-8. - PubMed
-
- Choi-Kwon S, Choi J, Kwon SU, Kang DW, Kim JS. Fluoxetine improves the quality of life in patients with post-stroke emotional disturbances. Cerebrovascular Diseases 2008;26:266-71. - PubMed
-
- Choi-Kwon S, Han SW, Kwon SU, Kang D, Kim CS, Kim JS. Fluoxetine treatment in poststroke depression, emotional incontinence, and anger proneness: a double-blind, placebo-controlled study. Stroke 2006;37:156-61. - PubMed
-
- Choi-Kwon S, Kwon SU, Kang DW, Kim JS. Fluoxetine improves the quality of life in patients with post-stroke emotional disturbances. Stroke 2009;40:e282. - PubMed
Chollet 2011 {published data only}
-
- Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurology 2011;10:123-30. - PubMed
Clark 2003 {published data only}
-
- Clark MS, Rubenach S, Winsor A. A randomized controlled trial of an education and counselling intervention for families after stroke. Clinical Rehabilitation 2003;17(7):703-12. - PubMed
CTRI/2021/02/031410 {published data only}
-
- CTRI/2021/02/031410. Treatment of depression after stroke in Unani Medicine. http://www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=51277&... (first received 18 February 2021).
da SilvaJunior 2019 {published data only}
-
- da Silva Junior B, Fernandes H, Rassi M, Souza C, Maria A. Proceedings #4: Repetitive transcranial magnetic stimulation (rTMS) improves depressive symptoms and quality of life of post-stroke patients. Brain Stimulation 2019;12(2):e60-e62.
Delbari 2011 {published data only}
-
- Delbari A, Salman-Roghani R, Lokk J. Effect of methylphenidate and/or levodopa combined with physiotherapy on mood and cognition after stroke: a randomized, double-blind, placebo-controlled trial. European Neurology 2011;66:7-13. - PubMed
Doshi 2019 {published data only}
-
- Doshi K, Henderson SL, Sugumarb L, Lowc AY, Thilarajahc S, De Silva DA. A pilot study investigating the impact of mindfulness based interventions on the psychosocial well-being of stroke survivors. Journal of Neurological Sciences 2017;381:410.
-
- NCT03910855. Impact of mindfulness on psychological well-being of stroke survivors and their caregivers (SOMII). https://clinicaltrials.gov/ct2/show/NCT03910855 (first received 10 April 2019).
Downes 1995 {published data only}
-
- Downes B, Rooney V, Oyebode JR, Roper-Hall A, Mayer P, Main A. The effect of giving information and counselling on depression and anxiety in stroke survivors and carers (The Birmingham Stroke Counselling Project). Unpublished 1995.
EUCTR2005‐005266‐37‐DE {published data only}
-
- EUCTR2005-005266-37-DE. Influence of Escitalopram on the incidence of depression and dementia following acute middle cerebral artery territory infarction. A randomized, placebo-controlled, double blind study. https://www.clinicaltrialsregister.eu/ctr-search/trial/2005-005266-37/DE (first received 4 January 2006).
EUCTR2014‐000846‐32‐ES {published data only}
-
- EUCTR2014-000846-32-ES. Effect of serotonin and levodopa on functional recovery in patients with cerebral infarction. https://clinicaltrials.gov/ct2/show/NCT02386475 (first received 12 March 2015).
Evans 1997 {published data only}
-
- Evans M, Hammond M, Wilson K, Lye M, Copeland J. Treatment of depression in the elderly: effect of physical illness on response. International Journal of Geriatric Psychiatry 1997;12:1189-94. - PubMed
Finkenzeller 2006 {published data only}
-
- Finkenzeller W, Zobel I, Rietz S, Schramm E, Berger M. Interpersonal psychotherapy and pharmacotherapy for post-stroke depression. Feasibility and effectiveness. Der Nervenarzt 2009;80:805-12. - PubMed
Franco 2001 {published data only}
-
- Franco K, Malhotra S, Robinson RG. Poststroke depression (multiple letters). American Journal of Psychiatry 2001;158(4):658‐60. - PubMed
Frey 2020 {published data only}
-
- Adcock A, Frey J, Mahoney J, Engler-Chiurazzi L, Najib U, Galster S, et al. Accelerated RTMS as a treatment for post-stroke depression in the subacute phase: an RCT. Neuromodulation 2019;22:E87.
-
- NCT04093843. TMS for post stroke depression. https://clinicaltrials.gov/ct2/show/NCT04093843 (first received 18 September 2019).
Gamberini 2021 {published data only}
-
- Gamberini G, Masuccio FG, Ferriero G, Cattaneo D, Solaro C. Safety and efficacy of vortioxetine on depressive symptoms and cognition in post-stroke patients: a pilot study. Journal of Affective Disorders 2021;286(2021):108-9. - PubMed
Griffin‐Musick 2020 {published data only}
-
- Griffin-Musick JR, Off CA, Kincheloe H, Kozlowski A, ML. The impact of a university-based Intensive Comprehensive Aphasia Program (ICAP) on psychosocial well-being in stroke survivors with aphasia. Aphasiology 2020;35(10):1363-89.
Gustafsson 2020 {published data only}
-
- Gustafsson L, Cornwell P, Kuys S, Hodson T, Thompson L, Wong A. Effectiveness of a telehealth self-management program for people with mild stroke: results of a randomised controlled trial with longitudinal follow-up. International Journal of Stroke 2020;15(1 Suppl):159.
Hadidi 2014 {published data only}
-
- Hadidi N, Buckwalter K, Lindquist R, Rangen C. Feasibility of a pilot study of problem-solving therapy for stroke survivors. Rehabilitation Nursing 2014;5:327-37. - PubMed
-
- Tierney M, Hadidi NN. Randomized feasibility study of problem-solving therapy compared to stroke education for depressive symptoms and quality of life in stroke survivor-care partner dyads. Circulation 2021;144 Suppl 1:11395.
He 2004 {published data only}
-
- He P, Kong Y, Xu L-Z. Randomized controlled observation on the effect of early application of fluoxetine in preventing depression after stroke. Chinese Journal of Clinical Rehabilitation 2004;8(28):6016-7.
He 2021 {published data only}
-
- He Y, Deng J, Zhang Y, Cai Z, Zhang H, Guo Y. The effect of fluoxetine on morning blood pressure surge in patients with ischemic stroke: a prospective preliminary clinical study. Blood Pressure Monitoring 2021;26:288-91. - PubMed
Hilari 2021 {published data only}
Hill 2019 {published data only}
Hjelle 2019 {published data only}
-
- Hjelle EG, Bragstad LK, Kirkevold M, Zucknick M, Bronken BA, Martinsen R, et al. Effect of a dialogue-based intervention on psychosocial well-being 6 months after stroke in Norway: a randomized controlled trial. Journal of Rehabilitation Medicine 2019;51:557-65. - PubMed
Hu 2003 {published data only}
-
- Hu Z, Hu Y, Lu Q. Impact of early rehabilitation therapy on post stroke depression. Chinese Journal of Clinical Rehabilitation 2003;3(15):1.
ISRCTN60046672 {published data only}
-
- ISRCTN60046672. Sertraline in post stroke depression in Irkutsk, Russia. https://www.isrctn.com/ISRCTN60046672 (first received 27 February 2012).
ISRCTN88489864 {published data only}
-
- ISRCTN88489864. Influence of a single dose of fluoxetine on muscle activation patterns and functional ability in chronic stroke patients. http://www.isrctn.com/ISRCTN88489864 (first received 23 July 2005).
Jiang 2004 {published data only}
-
- Jiang JB, Li GC. Effects of post-stroke depression and anti-depression therapy on rehabilitation of neurological function in patients with meta method. Chinese Journal of Clinical Rehabilitation 2004;8(31):6829-31.
Jorge 2004 {published data only}
-
- Jorge RE, Robinson RG, Tateno A, Narushima K, Acion L, Moser D, et al. Repetitive transcranial magnetic stimulation as treatment of poststroke depression: a preliminary study. Biological Psychiatry 2004;55:398-405. - PubMed
Jorge 2008 {published data only}
-
- Jorge RE, Moser DJ, Acion M, Robinson RG. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Archives of General Psychiatry 2008;65(3):268-76. - PubMed
JPRN‐UMIN000013200 {published data only}
-
- JPRN-UMIN000013200. Effects of escitalopram for post stroke depressive patients: evaluated by QIDS-J. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&ac... (first received 20 February 2014).
JPRN‐UMIN000027051 {published data only}
-
- JPRN-UMIN000027051. Clinical study of repetitive transcranial magnetic stimulation in stroke patient with apathy. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000031009 (first received 18 April 2017) (first received 18 April 2017).
JPRN‐UMIN000029117 {published data only}
-
- JPRN-UMIN000029117. The effect of cognitive rehabilitation vs. low intensity exercise for post-stroke depression in long-term care health facilities: a randomized controlled trial. https://rctportal.niph.go.jp/en/detail?trial_id=UMIN000029117 (first received 15 March 2018).
Kim 2010a {published data only}
-
- Kim BR, Kim D, Chun MH, Yi JH, Kwon JS. Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. American Journal of Physical Medicine and Rehabilitation 2010;89(5):362-8. - PubMed
Kim 2010b {published data only}
-
- Kim BR, Kim D, Chun MH, Yi JH, Kwon JS. Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. American Journal of Physical Medicine and Rehabilitation 2010;89(5):362-8. - PubMed
Kim 2017 {published data only}
-
- Kim JS, Lee E-J, Chang D, Park J-H, Ahn SH, Cha J-K, et al. Efficacy of early administration of escitalopram on depressive and emotional symptoms and neurological dysfunction after stroke: a multicentre, double-blind,randomised, placebo-controlled study. Lancet Psychiatry 2017;4(1):33-43. - PubMed
-
- Lee EJ, Kim JS, Chang DI, Park JH, Ahn SH, Cha JK, et al. Post-stroke depressive symptoms: varying responses to escitalopram by individual symptoms and lesion location. Journal of Geriatric Psychiatry and Neurology 2021;34(6):565-73. - PubMed
Kim 2017a {published data only}
Kim 2019 {published data only}
-
- Kim YW. Abstract #71: Effects of repetitive transcranial magnetic stimulation on cognition and neuroplasticity in subacute stroke patients. Brain Stimulation 2019;12(2):E25.
Kok 2021 {published data only}
-
- Kok RM, Veraart JKE. Li X & Zhang C. Comparative efficacy of nine antidepressants in treating Chinese patients with post-stroke depression: a network meta-analysis. Journal of Affective Disorders 2021;278:405-6. - PubMed
Konigsberg 2021 {published data only}
-
- Konigsberg A, Sehner S, Arlt S, Cheng BS, Simonsen CZ, Boutitie F, Investigators Wake-Up. Effect of intravenous alteplase on post-stroke depression in the WAKE UP trial. European Journal of Neurology 2021;28(6):2017-25. - PubMed
Kootker 2012 {published data only}
-
- Kootker JA, Rasquin SMC, Lem FC, Heughten CM, Fasotti L, Geurts ACH. Augmented cognitive behavioral therapy for poststroke depressive symptoms: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2017;98:687-94. - PubMed
-
- Kootker JA, Rasquin SMC, Smits P, Geurts AC, Heugten CM, Fasotti L. An augmented cognitive behavioural therapy for treating post-stroke depression: description of a treatment protocol. Clinical Rehabilitation 2015;29(9):833-43. - PubMed
Laska 2005 {published data only}
-
- Laska AC, Arbin M, Kahan T, Hellblom A, Murray V. Long-term antidepressant treatment with moclobemide for aphasia in acute stroke patients: a randomised, double-blind, placebo-controlled study. Cerebrovascular Diseases 2005;19(2):125-32. - PubMed
Leijon 1989 {published data only}
-
- Leijon G, Boivie J. Central post-stroke pain: a controlled trial of amitriptyline and carbamazepine. Pain 1989;36(1):27-36. - PubMed
Li 2016 {published data only}
-
- Li C, Chen X-H, Liang H. Transcranial magnetic stimulation to explore the mechanism of treatment of stagnation of liver-Qi type post-stroke depression. China & Foreign Medical Treatment 2016;34:175-7.
Li 2021 {published data only}
Liang 2003 {published data only}
-
- Liang Z, Tang S, Liu J. Clinical efficacy of fluoxetine in treatment of patients with depression after acute stroke. Chinese Journal of Clinical Rehabilitation 2003;7(13):1924-5.
Lobjanidze 2010 {published data only}
-
- Lobjanidze N, Dzagnidze A, Jeiranashvili A, Kukava M, Beridze M, Khachiashvili M, et al. Long-term effects of the use of citicoline (ceraxone) in the post-stroke cognitive-mood impairment. Cerebrovascular Diseases 2010;5(1):14.
Majumdar 2019 {published data only}
-
- Majumdar S, Morris R. Brief group-based acceptance and commitment therapy for stroke survivors. British Journal of Clinical Psychology 2019;58(1):70-90. - PubMed
Mauri 1988 {published data only}
-
- Marui L, Arboix A, Marti-Vilalta JL. Efficacy of antidepressive treatment in affective disorders associated to ischemic vascular disease. Neurologia 1988;3 Suppl 3:10.
Meara 1998 {published data only}
-
- Meara JR. A randomised double blind placebo controlled study of the treatment of post stroke depression. National Research Register.
-
- Meara RJ, Thalanany M, Balonwu V, Hobson P. The treatment of depression after stroke with the selective serotonin reuptake inhibitor sertraline. Cerebrovascular Diseases 1998;8 Suppl 4:90.
Morariu 2019 {published data only}
-
- Morariu EA, Patrascu MC, Fainarea AF, Manolache RE, Alexandru IA. Effectiveness of the pharmacological approach in preventing post-stroke depression. European Neuropsychopharmacology 2019;29:s703‐s704.
Narushima 2007 {published data only}
-
- Narushima K, Paradiso S, Moser D, Jorge R, Robinson R. Effect of antidepressant therapy on executive function after stroke. British Journal of Psychiatry 2007;190:260-5. - PubMed
NCT00071643 {published data only}
-
- NCT00071643. Preventing post-stroke depression. https://clinicaltrials.gov/ct2/show/NCT00071643 (first received 30 October 2003).
NCT00177424 {published data only}
-
- NCT00177424. Sertraline may not be effective in preventing post-stroke depression. https://clinicaltrials.gov/ct2/show/NCT00177424 (first received 25 September 2005).
NCT02947776 {published data only}
-
- NCT02947776. Adjustment with aphasia after stroke: SUPERB. https://clinicaltrials.gov/ct2/show/NCT02947776 (first received 28 October 2016).
NCT03256305 {published data only}
-
- NCT03256305. A study of rTMS personalized precision treatment of post-stroke depression. https://clinicaltrials.gov/ct2/show/NCT03256305 (first received 19 March 2018).
NCT03500250 {published data only}
-
- NCT03500250. Feasibility of the post-stroke depression-toolkit. https://clinicaltrials.gov/ct2/show/NCT03500250 (first received 18 April 2018).
NCT03615079 {published data only}
-
- NCT03615079. Internet-based CBT after stroke pilot. https://clinicaltrials.gov/ct2/show/NCT03615079 (first received 3 August 2018).
NCT03750526 {published data only}
-
- NCT03750526. Effectiveness of augmented reality and repetitive transcranial magnetic stimulation. https://ClinicalTrials.gov/show/NCT03750526 (first received 23 November 2018).
NCT03761303 {published data only}
-
- NCT03761303. rTMS as an add-on therapy in patients with post-stroke depression. https://clinicaltrials.gov/ct2/show/NCT03761303 (first received 3 December 2018).
NCT03826875 {published data only}
-
- NCT03826875. Poststroke depression in hemorrhagic stroke. https://clinicaltrials.gov/ct2/show/NCT03826875 (first received 1 February 2019).
NCT03864484 {published data only}
-
- NCT03864484. iPad Application-based Intervention for post-stroke depression. https://ClinicalTrials.gov/show/NCT03864484 (first received 6 March 2019).
NCT03910855 {published data only}
-
- NCT03910855. Impact of mindfulness on psychological well-being of stroke survivors and their caregivers. https://clinicaltrials.gov/ct2/show/NCT03910855 (first received 10 April 2019).
NCT03956693 {published data only}
-
- NCT03956693. Helping ease anxiety and depression following stroke. https://ClinicalTrials.gov/show/NCT03956693 (first received 21 May 2019).
NCT04011202 {published data only}
-
- NCT04011202. Virtual reality, mood, and sedentary behaviour after stroke. https://ClinicalTrials.gov/show/NCT04011202 (first received 8 July 2019).
NCT04302493 {published data only}
-
- NCT04302493. Mindfulness based stress reduction and post-stroke cognition. https://clinicaltrials.gov/ct2/show/NCT04302493 (10 March 2020).
NCT04318951 {published data only}
-
- NCT04318951. Impact of intensive social interaction on post-stroke depression in individuals with aphasia: a proof-of-concept trial. https://clinicaltrials.gov/ct2/show/NCT04318951 (first received 24 March 2020).
NCT04567472 {published data only}
-
- NCT04567472. HEADS: UP online psychological self-management intervention: feasibility 2. https://clinicaltrials.gov/ct2/show/NCT04567472 (first received 28 September 2020).
NCT04655937 {published data only}
-
- NCT04655937. Wellbeing After Stroke (WAterS). https://ClinicalTrials.gov/show/NCT04655937 (first received 7 December 2020).
NCT04713020 {published data only}
-
- NCT04713020. Application of educational intervention on the knowledge, depression and self-efficacy of patients with cerebrovascular accident. https://www.clinicaltrials.gov/ct2/show/NCT04713020 (first received 19 January 2021).
NCT04776226 {published data only}
-
- NCT04776226. Post-stroke depression treatment effect on stroke recurrence. https://clinicaltrials.gov/ct2/show/NCT04776226 (first received 1 March 2021).
Niimi 2020 {published data only}
-
- Niimi M, Ishima T, Hashimoto K, Hara T, Yamada N, Abo M. Effect of repetitive transcranial magnetic stimulation on the kynurenine pathway in stroke patients. Neuroreport 2020;31(9):629-36. - PubMed
Ohtomo 1985 {published data only}
-
- Ohtomo E, Kutsuzawa T, Araki G, Hirai S, Terashi A, Kuzuya F, et al. Clinical usefulness of tiapride on psychiatric symptoms caused by cerebrovascular disorders. Clinical Evaluation 1985;13:295-332.
Ostwald 2014 {published data only}
-
- NCT00178529. Intervention for stroke survivors and their spousal caregivers. https://clinicaltrials.gov/ct2/show/NCT00178529 (first received 19 July 2001).
-
- Ostwald S, Godwin K, Cron S, Kelley C, Hersch G, Davis S. Home-based psychoeducational and mailed information programs for stroke-caregiving dyads post-discharge: a randomized trial. Disability and Rehabilitation 2014;36(1):55-62. - PubMed
Otomo 1986 {published data only}
-
- Otomo E, Tohgi H, Hirai S, Gotoh F, Hasegawa K, Tazaki Y, et al. Clinical evaluation of YM-08054 (indeloxazine) in the treatment of cerebrovascular disorder. Igaku no Ayumi 1986;136(7):535-55.
Poalelungi 2020 {published data only}
-
- Poalelungi A, Turiac E, Tulba D, Stoian D, Popescu BO. Remote ischemic conditioning might improve cognition, depression and infarct volume in acute ischemic stroke. International Journal of Stroke 2020;15(1 Suppl):561.
Raffaele 1996 {published data only}
-
- Raffaele R, Rampello L, Vecchio I, Tornali C, Malaguanera M. Trazodone therapy of the post-stroke depression. Archives of Gerontology and Geriatrics 1996;22 Suppl 1:217-20. - PubMed
Rich 2016 {published data only}
Robinson 2000 {published data only}
-
- Robinson RG, Schultz SK, Castillo C, Kopel T, Kosier T, Newman M, et al. Nortriptyline versus fluoxetine in the treatment of depression and in-short-term recovery after stroke: a placebo-controlled, double-blind study. American Journal of Psychiatry 2000;157(3):351-9. - PubMed
Robinson 2017 {published data only}
-
- Robinson RG, Jorge RE, Long J. Prevention of poststroke mortality using problem-solving therapy or escitalopram. American Journal of Geriatric Psychiatry 2017;25(5):512-9. - PubMed
Rudberg 2017 {published data only}
-
- Rudberg AS, Isaksson E, Lundstrom E. Efficacy of fluoxetine: a randomised controlled trial in stroke, the effects study in Sweden. European Stroke Journal 2017;2(1 Suppl 1):320-1.
Sieger 2018 {published data only}
-
- Sieger FS, Poveda MYP, Moreno AP, Sanchez JAM, Romero LAL, Guzman LCR, et al. Effects of repetitive transcranial magnetic stimulation in aphasic stroke: a randomised double-blind controlled clinical trial. Neurology 2018;90(15 Suppl 1):P5.004.
Sivenius 2001 {published data only}
-
- Sivenius J, Sarasoja T, Aaltonen H, Heinonen E, Kilkku O, Reinikainen K. Selegiline treatment facilitates recovery after stroke. Journal of Neurologic Rehabilitation 2001;15(3):183-90. - PubMed
Slenders 2019 {published data only}
-
- Slenders J, Van Den Berg-Vos R, Van Heugten C, Visser-Meily J, Kwa V. Screening and patient-tailored care for emotional and cognitive complaints in patients discharged home after ischemic stroke (eco-stroke trial). European Stroke Journal 2019;4:807.
Sonis 2004 {published data only}
-
- Sonis J. Mortality and poststroke depression. American Journal of Psychiatry 2004;16(1):1506-7. - PubMed
Su 2004a {published data only}
-
- Su XL, Xiao XC. Effect of psychotherapy on the motor functional rehabilitation in patients with post-stroke depression. Chinese Journal of Clinical Rehabilitation 2004;8(19):3720-1.
Sun 2000 {published data only}
-
- Sun B, Chan JL. Effects of psychological rehabilitation on the recovery of hemiplegia patients after stroke. Modern Rehabilitation 2000;4(1):36-7.
Szepfalusi 2017 {published data only}
-
- Szepfalusi N, Nemeth V, Kazinczi C, Holczer A, Vekony T, Csifcsak G, et al. The use of transcranial direct current stimulation in stroke rehabilitation: effects on mood and cognition. Brain Stimulation 2019;12(2):561-2.
-
- Szepfalusi N, Nemeth VL, Vekony T, Imre N, Balogh R, Holczer A, et al. Ten-day cognitive training program combined with transcranial direct current stimulation (tDCS) in stroke rehabilitation - a sham controlled study. European Neuropsychopharmacology 2017;27 Suppl 4:S1038-9.
-
- Szepfalusi N, Nemeth VL, Vekony T, Imre N, Balogh R, Holczer A, et al. The use of transcranial direct current stimulation (tDCS) combined with cognitive training in stroke rehabilitation - a sham-controlled pilot study. Cerebrovascular Diseases 2017;43 Suppl 1:61.
TCTR20181216001 {published data only}
-
- TCTR20181216001. Randomized controlled trial of fluoxetine or placebo on quality of life after acute ischemic stroke. https://www.thaiclinicaltrials.org/show/TCTR20181216001 (first received 16 December 2018).
Tian 2016 {published data only}
-
- Tian G. Sertraline hydrochloride treatment the clinical effect of the treatment of depression after stroke. The Medical Forum 2016;20:1606-7.
Uchida 2020 {published data only}
-
- NCT03864484. iPad application-based intervention for post-stroke depression. https://clinicaltrials.gov/ct2/show/NCT03864484 (first received 6 March 2019).
Visser 2015 {published data only}
-
- Visser MM, Heijenbrok-Kal MH, van't Spijker A, Busschbach JJV, Ribbers GM. Poster 44: problem solving therapy during outpatient rehabilitation for stroke: short-term results of a randomized controlled trial. Stroke 2014;Suppl 1:28. - PubMed
-
- Visser MM, Heijenbrok-Kal MH, van't Spijker A, Busschbach JJV, Ribbers GM. Problem solving therapy during outpatient stroke rehabilitation improves coping and HRQoL: a randomized controlled trial. Stroke 2015;1:1-28. - PubMed
Vranceanu 2020 {published data only}
-
- Vranceanu AM, Bannon S, Mace R, Lester E, Meyers E, Gates M, et al. Feasibility and efficacy of a resiliency intervention for the prevention of chronic emotional distress among survivor-caregiver dyads admitted to the Neuroscience Intensive Care Unit: a randomized clinical trial. JAMA Network Open 2020;3(10):16. - PMC - PubMed
Walker‐Batson 1995 {published data only}
-
- Walker-Batson D, Smith P, Curtis S, Unwin H, Greenlee R. Amphetamine paired with physical therapy accelerates motor recovery after stroke: further evidence. Stroke 1995;26(12):2254-9. - PubMed
Wang 2003 {published data only}
-
- Wang F, Dong X, Pan Y. Effect of yukangning in the treatment of post stroke depression and nerve function recovery. www.zglckf.com 2003;7(7):1225.
Wang 2009 {published data only}
-
- Wang P. Influence of magnetic stimulation to treat post stroke depression on recovery of neurological function. Chinese Nursing Research 2009;23(5):1356-7.
Wang 2020 {published data only}
-
- Wang XY, Li JY, Wang CW, Lv JQ. The effects of mindfulness-based intervention on quality of life and poststroke depression in patients with spontaneous intracerebral hemorrhage in China. International Journal of Geriatric Psychiatry 2020;35(5):572-80. - PubMed
Wu 2012 {published data only}
-
- Wu DY, Guo M, Gao YS, Kang YH, Guo JC, Jiang XL. Clinical effects of comprehensive therapy of early psychological intervention and rehabilitation training on neurological rehabilitation of patients with acute stroke. Asian Pacific Journal Tropical Medicine 2012;5:914-6. - PubMed
Xie 2005 {published data only}
-
- Xie R M, Liu JY, Quan HB, Wang DS, Luo M. A prospective random clinical contrast study of treatment with sertraline in elderly patients with post-stroke depression. Chinese Journal of Clinical Neurosciences 2005;13:294-7.
Xu 2010 {published data only}
-
- Xu H, Cao N, Xu D. Effect of cognitive behavioral psychological nursing on rehabilitation of post-stroke depression. Chinese Journal of Practical Nervous Diseases 2010;13:13-4.
Yao 2021 {published data only}
-
- Yao X-W, Yu Z-J, Mo C-Y, Pan H-S, Li C-Y, Li Y-L. The efficacy and safety of agomelatine, sertraline, and escitalopram for senile post-stroke depression: a randomized double-blind placebo-controlled trial. Clinical Neurology and Neurosurgery 2021;205:1-6. - PubMed
Ye 2004 {published data only}
-
- Ye LX, Wang H, Wang YD, Zhang L, Liang DS, Guo Y. Effect of anti-depressive therapy on the rehabilitation of psychological and neurological function after stroke. Chinese Journal of Cliical Rehabilitation 2004;8(31):6826-8.
Yu 2021 {published data only}
Zhang 2013a {published data only}
-
- Zhang Q. Effect of psychological nursing on neurological impairment and depression degree in patients with post-stroke depression. Chinese Journal of Practical Nervous Diseases 2013;16:83-4.
Zhou 2004 {published data only}
-
- Zhou CX, Su XL, Yang XZ, Xiao XC. Effect of psychological nursing on the rehabilitation of post-stroke depression. Chinese Journal of Clinical Rehabilitation 2004;8:3008-9.
References to studies awaiting assessment
Chen 2002a {published data only}
-
- Chen W, Wang GF, Chen XH, Sheng YL, Zhu H. Effects of paroxetine on function recovery in patients with poststroke depression. Chinese Journal of Clinical Rehabilitation 2002;6(13):2014-5.
Chen 2002b {published data only}
-
- Chen W, Wang GF, Chen XH, Sheng YL, Zhu H. Effects of paroxetine on function recovery in patients with poststroke depression. Chinese Journal of Clinical Rehabilitation 2002;6(13):2014-5.
Ding 2005 {published data only}
-
- Ding D, Zhang Z. Combination of paroxetine and psychotherapy in the treatment of post-stroke depression. Shanghai Archives of Psychiatry 2005;17(2):89-91.
Evans 1985 {published data only}
-
- Evans RL, Kleinman L, Halar EM, Herzer K. Predicting outcome of group counselling with severely disabled patients. American Journal of Physical Medicine 1985;64(1):24-31. - PubMed
Finkenzeller 2009 {published data only}
-
- Finkenzeller W, Zobel I, Rietz S, Schramm E, Berger M. Interpersonal psychotherapy and pharmacotherapy in post-stroke depression [Interpersonelle Psychotherapie und Pharmakotherapie bei Post-Stroke-Depression]. Nervenarzt 2009;80:805-12. - PubMed
Hanspal 2007 {published data only}
-
- Hanspal R. The effectiveness of sertraline in clinical management of depression with or without lability in brain-injured. National Research Register. [N0388126828]
He 2003 {published data only}
-
- He C. The effect of psychological intervention combined with amitriptyline on patients with depression after stroke. Chinese Journal of Clinical Rehabilitation 2003;7(5):850.
He 2005 {published data only}
-
- He Y. A clinical study of paroxetine joint psychotherapy in treating poststroke depression with anxiety. Chinese Journal of Practical Nervous Diseases 2006;9(1):34-5.
-
- He Y. Prospective study of effects of paroxetine with mental intervention on depression and anxiety after stroke. Nervous Disease and Mental Health 2005;5(1):6-8.
Huang 2005 {published data only}
-
- Huang C, Shi G, Bai C. A comparative study of venlafaxine plus cognitive therapy in the treatment of post-stroke depression. Chinese Journal of Behavioral Medical Science 2005;14(9):790-1.
IRCT201008214607N1 {published data only}
-
- IRCT201008214607N1. Comparative evaluation of effectiveness of combined cognitive behaviour and drug therapy and drug therapy on poststroke depression. https://en.irct.ir/trial/4919 (first received 10 October 2010).
Katz 1998 {published data only}
-
- Katz RA, Hubbard DJ, Blaine J. The effect of group psychotherapy on post-stroke depression. Rehabilitation Psychology 1998;43(2):178.
Kuriakose 2020 {published data only}
-
- Kuriakose B, Zhang J. Functional and physiological assessment of combined transcranial direct current stimulation (tDCS) and upper extremity training on acute stroke patient. https://pmrjabstracts.org/abstract/functional-and-physiological-assessme... (accessed 29 August 2021).
Latow 1983 {published data only}
-
- Latow J. Psychotherapy and its effect on depression, sick-role identification and rehabilitation outcome for stroke victims. Archives of Physical Medicine and Rehabilitation 1983;64(10):511-2.
Lee 2005 {published data only}
-
- Lee NG, Choi IS, Kim JH, Lee SY, Han JY. The effect of repetitive transcranial magnetic stimulation on the post-stroke depression. In: Proceedings of the Proceedings of 3rd World Congress of the International Society of Physical and Rehabilitation Medicine - ISPRM. Brazil, Sao Paulo, 10-14 April 2005:105-9.
Li 2019 {published data only}
-
- Li JM, Dai JQ, Tan YY, Gu JW. Effect of antidepressant therapy on neurological rehabilitation and neuron-specific enolase in elderly patients with post-stroke depression. Journal of the American Geriatrics Society 2019;67:S636.
Liu 2010 {published data only}
-
- Liu J, Li X. High- frequency transcranial magnetic stimulation for alleviating post-stroke depression. Chinese Journal of Physical Medical Rehabilitation 2010;32(7):513-5.
Pearson 2005 {published data only}
-
- Pearson V. Educational intervention reduces occurrence of depression in community-dwelling stroke survivors. Stroke 2005;36(2):423.
Razazian 2016 {published data only}
-
- Razazian N, Esmaeili O, Almasi A. Effect of fluoxetine on motor improvement in ischemic stroke patients: a double blind clinical trial study. Zahedan Journal of Research in Medical Sciences 2016;18(7):e7549.
Tang 2002 {published data only}
-
- Tang LY, Ye JH. Cognitive therapy on depression and cognitive dysfunction after stroke. Nursing Journal of Chinese People's Liberation Army 2002;19(4):6-7.
Wang 2015 {published data only}
-
- Wang J, Zhang M, Xu L. The clinical observation of post-stroke depression improvement by repetitive transcranial magnetic stimulation. Chinese Journal of Rehabilitation 2015;6(30):167-70.
Yan 2010a {published data only}
-
- Yan TT, He LM, Gu ZT. A randomized, controlled study of high- and low-frequency rTMS on the treatment outcome for post stroke depression [高频及低频重复经颅磁剌激对脑卒中后抑郁的疗效对比研究]. Qingdao Yiyao Weisheng 2010;42(2):81-5.
Yan 2010b {published data only}
-
- Yan TT, He LM, Gu ZT. A randomized, controlled study of high- and low-frequency rTMS on the treatment outcome for post stroke depression [高频及低频重复经颅磁剌激对脑卒中后抑郁的疗效对比研究]. Qingdao Yiyao Weisheng 2010;42(2):81-5.
Yan 2010c {published data only}
-
- Yan TT, He LM, Gu ZT. A randomized, controlled study of high- and low-frequency rTMS on the treatment outcome for post stroke depression [[高频及低频重复经颅磁剌激对脑卒中后抑郁的疗效对比研究]]. Qingdao Yiyao Weisheng 2010;42(2):81-5.
Yan 2010d {published data only}
-
- Yan TT, He LM, Gu ZT. A randomized, controlled study of high- and low-frequency rTMS on the treatment outcome for post stroke depression [高频及低频重复经颅磁剌激对脑卒中后抑郁的疗效对比研究]. Qingdao Yiyao Weisheng 2010;42(2):81-5.
Yu 2019 {published data only}
-
- Yu H-L, Cao D-X, Liu J. Effect of a novel designed intensive patient care program on cognitiveimpairment, anxiety, depression as well as relapse free survival in acuteischemic stroke patients: a randomized controlled study. Neurological Research 2019;41(9):857-866. - PubMed
Zhang 2021 {published data only}
-
- Zhang W, Li G, Song S. Study on the effect of rehabilitation nursing on patients with cerebra ischemic stroke. International Journal of Clinical and Experimental Medicine 2021;14(5):1943-9.
References to ongoing studies
ACTRN12620000165987 {published data only}
-
- ACTRN12620000165987. Examining the efficacy of an online cognitive behaviour therapy (CBT) - based self-management program for adults with neurological disorders. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379054&... (first received 31 January 2020).
ChiCTR1800020468 {published data only}
-
- ChiCTR1800020468. Therapeutic effect of high frequency repetitive transcranial magnetic stimulation with different frequencies on patients with post-stroke depression. https://www.chictr.org.cn/showprojen.aspx?proj=33127 (first received 31 December 2018).
ChiCTR1900024245 {published data only}
-
- ChiCTR1900024245. The effect of repeated transcranial magnetic stimulation on post-stroke depression and the and mechanism research by functional MRI. https://www.chictr.org.cn/showproj.aspx?proj=39878 (first received 7 February 2019).
ChiCTR1900025440 {published data only}
-
- ChiCTR1900025440. Effects of transcranial direct current stimulation for the treatment of post-stroke depression. https://www.chictr.org.cn/showproj.aspx?proj=42559 (first received 26 August 2019).
ChiCTR1900027686 {published data only}
-
- ChiCTR1900027686. The effect and mechanism of intermittent theta burst stimulation on post-stroke depression. https://www.chictr.org.cn/showproj.aspx?proj=45960 (first received 23 November 2019).
ChiCTR2000029809 {published data only}
-
- ChiCTR2000029809. Cognitive effects of electrical current therapy in post-stroke depression: a study protocol for a randomized controlled trial. https://www.chictr.org.cn/showproj.aspx?proj=38853 (first received 14 February 2020).
ChiCTR2000035582 {published data only}
-
- ChiCTR2000035582. Clinical study of low intensity ultrasound nerve stimulation in the treatment of post-stroke anxiety and depression. https://www.chictr.org.cn/historyversionpub.aspx?regno=ChiCTR2000035582 (first received 17 August 2020).
ChiCTR2100041707 {published data only}
-
- ChiCTR2100041707. Effects of rTMS on depressive state and motor function in patients with subthreshold depression after stroke. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2100041707 (first received 1 January 2021).
Ding 2021 {published data only}
IRCT20090716002195N3 {published data only}
-
- IRCT20090716002195N3. Efficacy of mindfulness-based intervention and transcranial direct current stimulation (TDCS) on cognitive disorders, and emotional problems in patients with stroke: a randomized clinical trial. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20090716002195N3 (first received 31 July 2019).
IRCT2017030921965N4 {published data only}
-
- IRCT2017030921965N4. Clinical trial to evaluate the efficacy of electrical stimulation of the brain with direct electrical current on depression after stroke. https://en.irct.ir/trial/19078 (first received 28 May 2017).
Kirkevold 2018 {published data only}
NCT03056287 {published data only}
-
- NCT03056287. Exercise and brain stimulation for post-stroke. https://clinicaltrials.gov/ct2/show/NCT03056287 (first received 17 February 2017).
NCT03645759 {published data only}
-
- NCT03645759. Improving quality of life for veterans with stroke and psychological distress. https://clinicaltrials.gov/ct2/show/NCT03645759 (first received 24 August 2018).
NCT04941482 {published data only}
-
- NCT04941482. Intervention effectiveness towards improving physical and mental health for post-stroke patients. https://clinicaltrials.gov/ct2/show/NCT04941482 (first received 28 June 2021).
NCT04985838 {published data only}
-
- NCT04985838. Helping Ease Anxiety and Depression following Stroke stage 3 (HEADS:UP). https://clinicaltrials.gov/ct2/show/NCT04985838 (first received 2 August 2021).
NCT05097040 {published data only}
-
- NCT05097040. A coach-guided online acceptance and commitment therapy (ACT) intervention for stroke survivors. https://ClinicalTrials.gov/show/NCT05097040 (first received 27 October 2021).
Tang 2017 {published data only}
Additional references
AFFINITY Trial Collaboration 2020
-
- AFFINITY Trial Collaboration. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurology 2020;19(8):651-60. - PubMed
Anderson 1995a
-
- Anderson CS, Linto J, Stewart-Wynne EG. A population-based assessment of the impact and burden of caregiving for long-term stroke survivors. Stroke 1995;26:843-9. - PubMed
APA 1987
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. Third edition. Washington, DC: American Psychiatric Association, 1987.
APA 1994
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Fourth edition. Washington, DC: American Psychiatric Association, 1994.
APA 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-V. Fifth edition. Arlington, VA: American Psychiatric Publishing, 2013.
Astrom 1996
-
- Astrom M. Generalized anxiety disorder in stroke patients: a 3-year longitudinal study. Stroke 1996;27:270-5. - PubMed
Ayerbe 2013
-
- Ayerbe L, Ayis S, Wolfe CDA, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. British Journal of Psychiatry 2013;202:14-21. - PubMed
Beck 1961
-
- Beck AT, Ward C, Mendelson M. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561-71. - PubMed
Blöchl 2019
-
- Blöchl M, Meissner S, Nestler S. Does depression after stroke negatively influence physical disability? A systematic review and meta-analysis of longitudinal studies. Journal of Affective Disorders 2019;247:45-56. - PubMed
Bucur 2018
-
- Bucur M, Papagno C. A systematic review of noninvasive brain stimulation for post-stroke depression. Journal of Affective Disorders 2018;238:69-78. - PubMed
Burton 2015
-
- Burton LJ, Tyson S. Screening for mood disorders after stroke: a systematic review of psychometric properties and clinical utility. Psychological Medicine 2015;45(1):29-9. - PubMed
Burvill 1995a
-
- Burvill PW, Johnson GA, Jamrozik KD, Anderson CS, Stewart-Wynne EG, Chakera TM. Prevalence of depression after stroke: the Perth Community Stroke Study. British Journal of Psychiatry 1995;166:320-7. - PubMed
Burvill 1996
-
- Burvill PW, Johnson GA, Chakera TMH, Stewart-Wynne EG, Anderson CS, Jamrozik KD. The place of site of lesion in the aetiology of post-stroke depression. Cerebrovascular Diseases 1996;6:208-15.
Burvill 1997
-
- Burvill P, Johnson G, Jamrozik KD, Anderson C. Risk factors for post-stroke depression. International Journal of Geriatric Psychiatry 1997;12:219-26. - PubMed
Carson 2000
-
- Carson AJ, MacHale S, Allen K, Lawrie SM, Dennis M, House A, et al. Depression after stroke and lesion location: a systematic review. Lancet 2000;356:122-6. - PubMed
Chan 2004
-
- Chan A, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. Journal of the American Medical Association 2004;291:2457-65. - PubMed
Chen 2006
-
- Chen Y, Guo JJ, Zhan S, Patel NC. Treatment effects of antidepressants in patients with post-stroke depression: a meta-analysis. Annals of Pharmacotherapy 2006;40(12):2115–22. - PubMed
Cipriani 2018
Deeks 2021
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Deutsch 1997
-
- Deutsch A, Braun S, Granger CV. The Functional Independence Measure (FIM Instrument). Journal of Rehabilitation Outcomes Measures 1997;1:67-71.
Ebrahim 1987a
-
- Ebrahim S, Barer D, Nouri F. Affective illness after stroke. British Journal of Psychiatry 1987;151:52-6. - PubMed
EFFECTS Trial Collaboration 2020
-
- EFFECTS Trial Collaboration. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurology 2020;19(8):661-9. - PubMed
FOCUS Trial Collaboration 2019
Folstein 1975
-
- Folstein MF, Folstein SE, McHugh PR. 'Mini-Mental State': a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189-98. - PubMed
Fournier 2010
Gill 2000
Goldberg 1972
-
- Goldberg DP. The detection of psychiatric illness by questionnaire. Maudsley Monograph No 21. Oxford: Oxford University Press, 1972.
Gompertz 1993
-
- Gompertz P, Pound P, Ebrahim S. The reliability of stroke outcome measurement. Clinical Rehabilitation 1993;7:290-6.
GRADEproGDT 2020 [Computer program]
-
- GRADEpro Guideline Development Tool. McMaster University, 2020 (developed by Evidence Prime, Inc.). Available from gradepro.org, 2020.
Hackett 2008a
Hackett 2014
-
- Hackett M, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. World Stroke Organisation 2014;9(December):1017-25. - PubMed
Hamilton 1960
Herrman 2022
-
- Herrman H, Patel V, Kieling C, Berk M, Buchweitz C, Cuijpers P, et al. Time for united action on depression: a Lancet-World Psychiatric Association Commission. Lancet 2022;399:957-1022. - PubMed
Herrmann 1998
-
- Herrmann N, Backe SE, Lawrence J, Szekely C, Szalai JP. The Sunnybrook stroke study: a prospective study of depressive symptoms and functional outcome. Stroke 1998;29:618-24. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
House 1987
-
- House A. Mood disorders after stroke: a review of the evidence. International Journal of Geriatric Psychiatry 1987;2:211-21.
House 1989
-
- House A, Dennis M, Hawton K, Warlow C. Methods of identifying mood disorders in stroke patients: experience in the Oxfordshire community stroke project. Age and Aging 1989;18:371-9. - PubMed
House 1991
-
- House A, Dennis M, Mogridge L, Warlow C, Hawton K, Jones L. Mood disorders in the year after first stroke. British Journal of Psychiatry 1991;158:83-92. - PubMed
House 2001
-
- House A, Knapp P, Bamford J, Vail A. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke 2001;32:696-701. - PubMed
Johnson 1991
-
- Johnson GA. Research into psychiatric disorder after stroke: the need for further studies. Australian & New Zealand Journal of Psychiatry 1991;25:358-70. - PubMed
Katona 1995
-
- Katona CLE, Watkin V. Depression in old age. Reviews in Clinical Gerontology 1995;5:427-41.
Keller 2003
-
- Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. Journal of the American Medical Association 2003;289(23):3152-60. - PubMed
Kirsch 2008
Ladwig 2018
-
- Ladwig S, Zhou Z, Xu Y, Wang X, Chow CK, Werheid K, et al. Comparison of treatment rates in depression after stroke versus myocardial infarction. A systematic review and meta-analysis of observational data. Psychosomatic Medicine 2018;80(8):754-63. - PubMed
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from: www.training.cochrane.org/handbook.
Legg 2021
Liao 2020
Lima 2001
Liu 2019
-
- Liu C, Wang M, Liang X, Xue J, Zhang G. Efficacy and safety of high-frequency repetitive transcranial magnetic stimulation for poststroke depression: a systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation 2019;100(10):1964-75. - PubMed
Lyden 2001
-
- Lyden PD, Lu M, Levine SR, Brott TG, Broderick J, the NINDS rtPA Stroke Study Group. A modified National Institutes of Health Stroke Scale for use in stroke clinical trials: preliminary reliability and validity. Stroke 2001;32:1310-7. - PubMed
Mahoney 1965
-
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:61-5. - PubMed
McCusker 1998
-
- McCusker J, Cole M, Keller E, Bellavance F, Berard A. Effectiveness of treatments of depression in older ambulatory patients. Archives of Internal Medicine 1998;158:705-12. - PubMed
Mead 2012
Mittmann 1997
-
- Mittmann N, Herrmann N, Einarson TR, Busto UE, Lanctot KL, Liu BA, et al. The efficacy, safety and tolerability of antidepressants in late life depression: a meta-analysis. Journal of Affective Disorders 1997;46:191-217. - PubMed
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382-9. - PubMed
Morris 1993b
-
- Morris PL, Robinson RG, Andrzejewski P, Samuels J, Price TR. Association of depression with 10-year poststroke mortality. American Journal of Psychiatry 1993;150:124-9. - PubMed
Normand 1999
-
- Normand ST. Meta-analysis: formulating, evaluating, combining, and reporting. Statistics in Medicine 1999;18:321-59. - PubMed
Paul 2006
-
- Paul SL, Dewey HM, Sturm JW, Macdonell RAL, Thrift AG. Prevalence of depression and use of antidepressant medication at 5-years poststroke in the North East Melbourne Stroke Incidence Study. Stroke 2006;37:2854-5. - PubMed
Rashid 2013
-
- Rashid T, Seligman MEP. Positive psychotherapy. In: Corsini RJ, Wedding D, editors(s). Current psychotherapies. 10 edition. Belmont, California, USA: Cengage, 2013:461–498.
Review Manager 2020 [Computer program]
-
- Review Manager (RevMan). Version 5.4.1. Copenhagen: The Cochrane Collaboration, 2020.
RevMan Web 2020 [Computer program]
-
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Robinson 1986
-
- Robinson RG, Bolla-Wilson K, Kaplan E, Lipsey JR, Price TR. Depression influences intellectual impairment in stroke patients. British Journal of Psychiatry 1986;148:541-7. - PubMed
Roth 2020
Schünemann 2021
-
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane 2021. Available from www.training.cochrane.org/handbook.
Sharpe 1990
-
- Sharpe M, Hawton K, House A, Molyneux A, Sandercock P, Bamford J, et al. Mood disorders in long-term survivors of stroke: associations with brain lesion location and volume. Psychological Medicine 1990;20:815-28. - PubMed
Shen 2017
-
- Shena XY, Liua MY, Chenga Y, Jiab C, Pana XY, Goua QY, et al. Repetitive transcranial magnetic stimulation for the treatment of poststroke depression: a systematic review and meta-analysis of randomized controlled clinical trials. Journal of Affective Disorders 2017;211:65-74. - PubMed
Snow 2000
-
- Snow V, Lascher S, Mottur-Pilson C, for the American College of Physicians-American Society of Internal Medicine. Pharmacologic treatment of acute major depression and dysthymia. Annals of Internal Medicine 2000;132(9):738-42. - PubMed
Stenager 1998
Towfighi 2017
-
- Towfighi A, Ovbiagele B, El Husseini N, Hackett ML, Jorge RE, Kissela BM, et al. Poststroke depression: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017;48:e30–e43. - PubMed
Turner 2012
-
- Turner A, Hambridge J, White J, Carter G, Clover K, Nelson L, Hackett M. Depression screening in stroke: a comparison of alternative measures with the SCID (Major Depressive Episode) as criterion standard. Stroke 2012;43:1000-5. - PubMed
Turner‐Stokes 2003
-
- Turner-Stokes L. Poststroke depression: getting the full picture. Lancet 2003;361(9371):1757-8. - PubMed
Verhagen 2001
-
- Verhagen AP, Vet HCW, Bie RA, Boers M, den Brandt PA. The art of quality assessment of RCTs included in systematic reviews. Journal of Clinical Epidemiology 2001;54:651-4. - PubMed
Wang 2018
Wilkinson 1997
-
- Wilkinson P. Cognitive therapy with elderly people. Age and Ageing 1997;26:53-8. - PubMed
Zigmond 1983
-
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67:361-70. - PubMed
Zimmerman 2002
-
- Zimmerman M, Mattia JI, Posternak MA. Are subjects in pharmacological treatment trials of depression representative of patients in routine clinical practice? American Journal of Psychiatry 2002;159(3):469-73. - PubMed
References to other published versions of this review
Hackett 2001
Hackett 2004
Hackett 2005
Hackett 2008
Hackett 2009
-
- Hackett ML, Anderson CS, House AO, Xia J. Interventions for treating depression after stroke. Stroke 2009;40:e487-e488. [DOI: 10.1161/STROKEAHA.109.547059] - DOI
Hackett 2020
-
- Allida S, Cox KL, Hsieh CF, Lang H, House A, Hackett ML. Pharmacological, psychological, and non-invasive brain stimulation interventions for treating depression after stroke. Cochrane Database of Systematic Reviews 2020, Issue 1. Art. No: CD003437. [DOI: 10.1002/14651858.CD003437.pub4] - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

