Engineering Tissue-Scale Properties with Synthetic Cells: Forging One from Many
- PMID: 37417657
- PMCID: PMC11017731
- DOI: 10.1021/acssynbio.3c00061
Engineering Tissue-Scale Properties with Synthetic Cells: Forging One from Many
Abstract
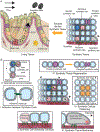
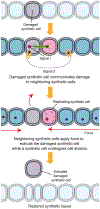
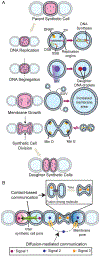
In metazoans, living cells achieve capabilities beyond individual cell functionality by assembling into multicellular tissue structures. These higher-order structures represent dynamic, heterogeneous, and responsive systems that have evolved to regenerate and coordinate their actions over large distances. Recent advances in constructing micrometer-sized vesicles, or synthetic cells, now point to a future where construction of synthetic tissue can be pursued, a boon to pressing material needs in biomedical implants, drug delivery systems, adhesives, filters, and storage devices, among others. To fully realize the potential of synthetic tissue, inspiration has been and will continue to be drawn from new molecular findings on its natural counterpart. In this review, we describe advances in introducing tissue-scale features into synthetic cell assemblies. Beyond mere complexation, synthetic cells have been fashioned with a variety of natural and engineered molecular components that serve as initial steps toward morphological control and patterning, intercellular communication, replication, and responsiveness in synthetic tissue. Particular attention has been paid to the dynamics, spatial constraints, and mechanical strengths of interactions that drive the synthesis of this next-generation material, describing how multiple synthetic cells can act as one.
Keywords: adhesion; cell mechanics; intercellular communication; regeneration; synthetic cells; tissue.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Wegst UGK; Bai H; Saiz E; Tomsia AP; Ritchie RO Bioinspired Structural Materials. Nat. Mater 2015, 14 (1), 23–36. - PubMed
-
- Sharma A; Arya SK Bio-Inspired Self-Healable Materials. In Self-Healing Smart Materials and Allied Applications; John Wiley & Sons, Ltd, 2021; pp 435–474. DOI: 10.1002/9781119710219.ch18. - DOI
-
- Ivanov I; Castellanos SL; Balasbas S; Otrin L; Marušič N; Vidaković-Koch T; Sundmacher K Bottom-Up Synthesis of Artificial Cells: Recent Highlights and Future Challenges. Annu. Rev. Chem. Biomol. Eng 2021, 12 (1), 287–308. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

