Advances in cochlear gene therapies
- PMID: 37417821
- PMCID: PMC10771539
- DOI: 10.1097/MOP.0000000000001273
Advances in cochlear gene therapies
Abstract
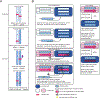
Purpose of review: Hearing loss is the most common sensory deficit and in young children sensorineural hearing loss is most frequently genetic in etiology. Hearing aids and cochlear implant do not restore normal hearing. There is significant research and commercial interest in directly addressing the root cause of hearing loss through gene therapies. This article provides an overview of major barriers to cochlear gene therapy and recent advances in preclinical development of precision treatments of genetic deafness.
Recent findings: Several investigators have recently described successful gene therapies in many common forms of genetic hearing loss in animal models. Elegant strategies that do not target a specific pathogenic variant, such as mini gene replacement and mutation-agnostic RNA interference (RNAi) with engineered replacement, facilitate translation of these findings to development of human therapeutics. Clinical trials for human gene therapies are in active recruitment.
Summary: Gene therapies for hearing loss are expected to enter clinical trials in the immediate future. To provide referral for appropriate trials and counseling regarding benefits of genetic hearing loss evaluation, specialists serving children with hearing loss such as pediatricians, geneticists, genetic counselors, and otolaryngologists should be acquainted with ongoing developments in precision therapies.
Copyright © 2023 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
RJHS directs the Molecular Otolaryngology and Renal Research Laboratories, which developed and offers comprehensive genetic testing for patients with hearing loss, and is a cofounder of Akouos, which develops gene therapies for genetic hearing loss.
Figures
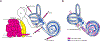
Similar articles
-
The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model.Health Technol Assess. 2009 Sep;13(44):1-330. doi: 10.3310/hta13440. Health Technol Assess. 2009. PMID: 19799825
-
Hearing Instruments for Unilateral Severe-to-Profound Sensorineural Hearing Loss in Adults: A Systematic Review and Meta-Analysis.Ear Hear. 2016 Sep-Oct;37(5):495-507. doi: 10.1097/AUD.0000000000000313. Ear Hear. 2016. PMID: 27232073 Free PMC article.
-
Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children.Cochrane Database Syst Rev. 2005 Jan 25;(1):CD001801. doi: 10.1002/14651858.CD001801.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2010 Oct 06;(10):CD001801. doi: 10.1002/14651858.CD001801.pub3. PMID: 15674886 Updated.
-
Chronic Electro-Acoustic Stimulation May Interfere With Electric Threshold Recovery After Cochlear Implantation in the Aged Guinea Pig.Ear Hear. 2024 Nov-Dec 01;45(6):1554-1567. doi: 10.1097/AUD.0000000000001545. Epub 2024 Jul 12. Ear Hear. 2024. PMID: 38992863 Free PMC article.
-
Bilateral versus unilateral hearing aids for bilateral hearing impairment in adults.Cochrane Database Syst Rev. 2017 Dec 19;12(12):CD012665. doi: 10.1002/14651858.CD012665.pub2. Cochrane Database Syst Rev. 2017. PMID: 29256573 Free PMC article.
Cited by
-
Single Administration of AAV-mAtp6v1b2 Gene Therapy Rescues Hearing and Vestibular Disorders Caused by Atp6v1b2-Induced Lysosomal Dysfunction in Hair Cells.Adv Sci (Weinh). 2025 Aug;12(29):e2408878. doi: 10.1002/advs.202408878. Epub 2025 Mar 11. Adv Sci (Weinh). 2025. PMID: 40068100 Free PMC article.
-
Vernonia amygdalina displays otoprotective effects via antioxidant pathway on cisplatin-induced hair cell loss in zebrafish.Arch Toxicol. 2025 Jul;99(7):2993-3005. doi: 10.1007/s00204-025-04038-8. Epub 2025 Mar 30. Arch Toxicol. 2025. PMID: 40159305
-
Gene Therapy for Inherited Hearing Loss: Updates and Remaining Challenges.Audiol Res. 2023 Dec 4;13(6):952-966. doi: 10.3390/audiolres13060083. Audiol Res. 2023. PMID: 38131808 Free PMC article. Review.
-
Genome-Wide Association Study of Age-Related Hearing Loss in CFW Mice Identifies Multiple Genes and Loci, Including Prkag2.J Assoc Res Otolaryngol. 2025 May 21. doi: 10.1007/s10162-025-00994-1. Online ahead of print. J Assoc Res Otolaryngol. 2025. PMID: 40399499
References
-
- Smith RJ, Bale JF, Jr., White KR. Sensorineural hearing loss in children. Lancet. 2005;365(9462):879–90. - PubMed
-
- Schmuziger N, Veraguth D, Probst R. [Universal newborn hearing screening--a silent revolution]. Praxis (Bern 1994). 2008;97(19):1015–21. - PubMed
-
-
Shearer AE, Hildebrand MS, Smith RJH. Hereditary Hearing Loss and Deafness Overview. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, et al., editors. GeneReviews((R)). Seattle (WA) 1993.
*Interested readers are invited to review this overview of the evaluation, differential diagnosis, and management of hereditary hearing loss.
-
-
- Niskar AS, Kieszak SM, Holmes A, et al. Prevalence of hearing loss among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey. JAMA. 1998;279(14):1071–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

