Long-term changes in heart rate and electrical remodeling contribute to alternans formation in heart failure: a patient-specific in silico study
- PMID: 37417871
- PMCID: PMC11575914
- DOI: 10.1152/ajpheart.00220.2023
Long-term changes in heart rate and electrical remodeling contribute to alternans formation in heart failure: a patient-specific in silico study
Abstract
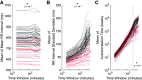
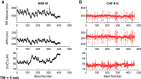
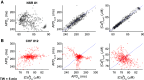
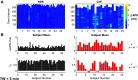
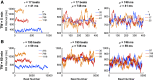
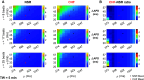
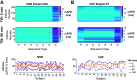
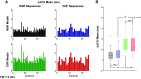
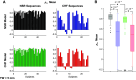
Individuals with chronic heart failure (CHF) have an increased risk of ventricular arrhythmias, which has been linked to pathological cellular remodeling and may also be mediated by changes in heart rate. Heart rate typically fluctuates on a timescale ranging from seconds to hours, termed heart rate variability (HRV). This variability is reduced in CHF, and this HRV reduction is associated with a greater risk for arrhythmias. Furthermore, variations in heart rate influence the formation of proarrhythmic alternans, a beat-to-beat alternation in the action potential duration (APD), or intracellular calcium (Ca). In this study, we investigate how long-term changes in heart rate and electrical remodeling associated with CHF influence alternans formation. We measure key statistical properties of the RR-interval sequences from ECGs of individuals with normal sinus rhythm (NSR) and CHF. Patient-specific RR-interval sequences and synthetic sequences (randomly generated to mimicking these statistical properties) are used as the pacing protocol for a discrete time-coupled map model that governs APD and intracellular Ca handling of a single cardiac myocyte, modified to account for pathological electrical remodeling in CHF. Patient-specific simulations show that beat-to-beat differences in APD vary temporally in both populations, with alternans formation more prevalent in CHF. Parameter studies using synthetic sequences demonstrate that increasing the autocorrelation time or mean RR-interval reduces APD alternations, whereas increasing the RR-interval standard deviation leads to higher alternans magnitudes. Importantly, we find that although both the CHF-associated changes in heart rate and electrical remodeling influence alternans formation, variations in heart rate may be more influential.NEW & NOTEWORTHY Using patient-specific data, we show that both the changes in heart rate and electrical remodeling associated with chronic heart failure influence the formation of proarrhythmic alternans in the heart.
Keywords: alternans; computational model; electrical remodeling; heart failure; heart rate variability.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, , et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 145: e153–e639, 2022. [Erratum in Circulation 146: e141, 2022]. doi: 10.1161/CIR.0000000000001052. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

