Torsional and strain dysfunction precede overt heart failure in a mouse model of dilated cardiomyopathy pathogenesis
- PMID: 37417875
- PMCID: PMC10538988
- DOI: 10.1152/ajpheart.00130.2023
Torsional and strain dysfunction precede overt heart failure in a mouse model of dilated cardiomyopathy pathogenesis
Abstract
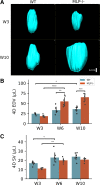
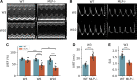
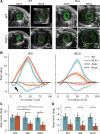
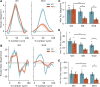
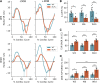
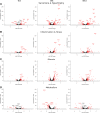
Detailed assessments of whole heart mechanics are crucial for understanding the consequences of sarcomere perturbations that lead to cardiomyopathy in mice. Echocardiography offers an accessible and cost-effective method of obtaining metrics of cardiac function, but the most routine imaging and analysis protocols might not identify subtle mechanical deficiencies. This study aims to use advanced echocardiography imaging and analysis techniques to identify previously unappreciated mechanical deficiencies in a mouse model of dilated cardiomyopathy (DCM) before the onset of overt systolic heart failure (HF). Mice lacking muscle LIM protein expression (MLP-/-) were used to model DCM-linked HF pathogenesis. Left ventricular (LV) function of MLP-/- and wild-type (WT) controls were studied at 3, 6, and 10 wk of age using conventional and four-dimensional (4-D) echocardiography, followed by speckle-tracking analysis to assess torsional and strain mechanics. Mice were also studied with RNA-seq. Although 3-wk-old MLP-/- mice showed normal LV ejection fraction (LVEF), these mice displayed abnormal torsional and strain mechanics alongside reduced β-adrenergic reserve. Transcriptome analysis showed that these defects preceded most molecular markers of HF. However, these markers became upregulated as MLP-/- mice aged and developed overt systolic dysfunction. These findings indicate that subtle deficiencies in LV mechanics, undetected by LVEF and conventional molecular markers, may act as pathogenic stimuli in DCM-linked HF. Using these analyses in future studies will further help connect in vitro measurements of the sarcomere function to whole heart function.NEW & NOTEWORTHY A detailed study of how perturbations to sarcomere proteins impact whole heart mechanics in mouse models is a major yet challenging step in furthering our understanding of cardiovascular pathophysiology. This study uses advanced echocardiographic imaging and analysis techniques to reveal previously unappreciated subclinical whole heart mechanical defects in a mouse model of cardiomyopathy. In doing so, it offers an accessible set of measurements for future studies to use when connecting sarcomere and whole heart function.
Keywords: cardiac mechanics; dilated cardiomyopathy; heart failure; muscle LIM protein; speckle-tracking echocardiography.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
Subtle abnormalities in contractile function are an early manifestation of sarcomere mutations in dilated cardiomyopathy.Circ Cardiovasc Genet. 2012 Oct 1;5(5):503-10. doi: 10.1161/CIRCGENETICS.112.962761. Epub 2012 Sep 4. Circ Cardiovasc Genet. 2012. PMID: 22949430 Free PMC article.
-
Speckle tracking echocardiography in hypokinetic non-dilated cardiomyopathy: comparison with dilated cardiomyopathy.ESC Heart Fail. 2020 Aug;7(4):1909-1916. doi: 10.1002/ehf2.12764. Epub 2020 May 19. ESC Heart Fail. 2020. PMID: 32424892 Free PMC article.
-
Sarcomere-based genetic enhancement of systolic cardiac function in a murine model of dilated cardiomyopathy.Int J Cardiol. 2018 Dec 15;273:168-176. doi: 10.1016/j.ijcard.2018.09.073. Epub 2018 Sep 21. Int J Cardiol. 2018. PMID: 30279005 Free PMC article.
-
Utility of strain imaging in conjunction with heart failure stage classification for heart failure patient management.J Echocardiogr. 2019 Mar;17(1):17-24. doi: 10.1007/s12574-018-0408-2. Epub 2018 Nov 15. J Echocardiogr. 2019. PMID: 30443873 Review.
-
Risk Stratification of Genetic, Dilated Cardiomyopathies Associated With Neuromuscular Disorders: Role of Cardiac Imaging.Circulation. 2018 Jun 5;137(23):2514-2527. doi: 10.1161/CIRCULATIONAHA.117.031110. Circulation. 2018. PMID: 29866775 Review.
Cited by
-
Comparative mechanistic analysis of danicamtiv and omecamtiv mecarbil's in vivo cardiac effects.J Gen Physiol. 2025 Jul 7;157(4):e202513762. doi: 10.1085/jgp.202513762. Epub 2025 Jun 18. J Gen Physiol. 2025. PMID: 40531056
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

