Randomized phase III trial of metabolic imaging-guided dose escalation of radio-chemotherapy in patients with newly diagnosed glioblastoma (SPECTRO GLIO trial)
- PMID: 37417948
- PMCID: PMC10768994
- DOI: 10.1093/neuonc/noad119
Randomized phase III trial of metabolic imaging-guided dose escalation of radio-chemotherapy in patients with newly diagnosed glioblastoma (SPECTRO GLIO trial)
Abstract
Background: Glioblastoma (GBM) systematically recurs after a standard 60 Gy radio-chemotherapy regimen. Since magnetic resonance spectroscopic imaging (MRSI) has been shown to predict the site of relapse, we analyzed the effect of MRSI-guided dose escalation on overall survival (OS) of patients with newly diagnosed GBM.
Methods: In this multicentric prospective phase III trial, patients who had undergone biopsy or surgery for a GBM were randomly assigned to a standard dose (SD) of 60 Gy or a high dose (HD) of 60 Gy with an additional simultaneous integrated boost totaling 72 Gy to MRSI metabolic abnormalities, the tumor bed and residual contrast enhancements. Temozolomide was administered concomitantly and maintained for 6 months thereafter.
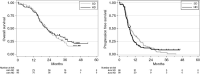
Results: One hundred and eighty patients were included in the study between March 2011 and March 2018. After a median follow-up of 43.9 months (95% CI [42.5; 45.5]), median OS was 22.6 months (95% CI [18.9; 25.4]) versus 22.2 months (95% CI [18.3; 27.8]) for HD, and median progression-free survival was 8.6 (95% CI [6.8; 10.8]) versus 7.8 months (95% CI [6.3; 8.6]), in SD versus HD, respectively. No increase in toxicity rate was observed in the study arm. The pseudoprogression rate was similar across the SD (14.4%) and HD (16.7%) groups. For O(6)-methylguanine-DNA methyltransferase (MGMT) methylated patients, the median OS was 38 months (95% CI [23.2; NR]) for HD patients versus 28.5 months (95% CI [21.1; 35.7]) for SD patients.
Conclusion: The additional MRSI-guided irradiation dose totaling 72 Gy was well tolerated but did not improve OS in newly diagnosed GBM.
Trial registration: NCT01507506; registration date: December 20, 2011. https://clinicaltrials.gov/ct2/show/NCT01507506?cond=NCT01507506&rank=1.
Keywords: 3D magnetic resonance spectroscopic imaging; clinical trial; glioblastoma; phase III; radiotherapy.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Comment in
-
SPECTRO GLIO trial aftermath: Where do we go from here?Neuro Oncol. 2024 Jan 5;26(1):164-165. doi: 10.1093/neuonc/noad166. Neuro Oncol. 2024. PMID: 37675932 Free PMC article. No abstract available.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Ling CC, Humm J, Larson S, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47(3):551–560. - PubMed
-
- Laprie A, Catalaa I, Cassol E, et al. Proton magnetic resonance spectroscopic imaging in newly diagnosed glioblastoma: predictive value for the site of postradiotherapy relapse in a prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2008;70(3):773–781. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

