Does timing of neoadjuvant chemotherapy influence the prognosis in patients with early triple negative breast cancer?
- PMID: 37418056
- PMCID: PMC10465651
- DOI: 10.1007/s00432-023-05060-y
Does timing of neoadjuvant chemotherapy influence the prognosis in patients with early triple negative breast cancer?
Abstract
Purpose: For patients with triple negative breast cancer (TNBC), the optimal time to initiate neoadjuvant chemotherapy (TTNC) is unknown. This study evaluates the association between TTNC and survival in patients with early TNBC.
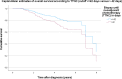
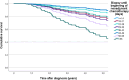
Methods: A retrospective study using data from of a cohort of TNBC patients diagnosed between January 1, 2010 to December 31, 2018 registered in the Tumor Centre Regensburg was performed. Data included demographics, pathology, treatment, recurrence, and survival. Interval to treatment was defined as days from pathology diagnosis of TNBC to first dose of neoadjuvant chemotherapy (NACT). The Kaplan-Meier and Cox regression methods were used to evaluate the impact of TTNC on overall survival (OS) and 5 year OS.
Results: A total of 270 patients were included. Median follow up was 3.5 years. The 5-year OS estimates according to TTNC were 77.4%, 66.9%, 82.3%, 80.6%, 88.3%, 58.3%, 71.1% and 66.7% in patients who received NACT within 0-14, 15-21, 22-28, 29-35, 36-42, 43-49, 50-56 and > 56 days after diagnosis. Patients who received systemic therapy early had the highest estimated mean OS of 8.4 years, while patients who received systemic therapy after more than 56 days survived an estimated 3.3 years.
Conclusion: The optimal time interval between diagnosis and NACT remains to be determined. However, starting NACT more than 42 days after diagnosis of TNBC seems to reduce survival. Therefore, it is strongly recommended to carry out the treatment in a certified breast center with appropriate structures, in order to enable an adequate and timely care.
Keywords: Clinical cancer registry data; Neoadjuvant chemotherapy; Time to systemic therapy; Triple negative breast cancer.
© 2023. The Author(s).
Conflict of interest statement
Miriam Pigerl, Sophie Räpple, Verena Zeltner, Peter Ugocsai, Elisabeth Inwald, Michael Gerken, Miriam Fernandez and Monika Klinkhammer-Schalke declare they have no financial interests. Maria Hatzipanagiotou has received Honoraria for lectures and/or consulting from Novartis, Lilly, Roche, Pfizer and AstraZeneca. Madeleine Hetterich has received speaker honoraria from Celgene, Novartis, MSD, GSK. Eisai and AstraZeneca as well as author honoraria from Thieme and Elsevier and travel reimbursement from Novartis. Olaf Ortmann is on the board of the German Cancer society. He received speaker honoraria from MSD SHARP & DOHME GMBH. Verband Forschender Arzneimittelhersteller vfa, Novo Nordisk, AstraZeneca, Aurikamed, Med Update, RG Ärztefortbildung, Pierre Fabre Pharma GmbH and holds stocks from Bayer, Novartis, Curevac and Fresenius. Stephan Seitz has received speaker honoraria from AstraZeneca, GE, GSK, IGEA, Lilly, MSD, Novartis, Pfizer, Roche and honoraria for consulting from AstraZeneca, GSK, Lilly, MSD, Novartis, Pfizer and Roche.
Figures
References
-
- Biagi JJ, Raphael M, King WD et al (2011) The effect of delay in time to adjuvant chemotherapy (TTAC) on survival in breast cancer (BC). A systematic review and meta-analysis. JCO 29:1128. 10.1200/jco.2011.29.15_suppl.1128
-
- Burstein HJ, Curigliano G, Loibl S et al (2019) Estimating the benefits of therapy for early-stage breast cancer. The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol 30:1541–1557. 10.1093/annonc/mdz235 - PubMed
-
- Chaudhary LN, Wilkinson KH, Kong A (2018) Triple-negative breast cancer who should receive neoadjuvant chemotherapy? Surg Oncol Clin North Am 27:141–153. 10.1016/j.soc.2017.08.004 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical