IgA nephropathy in adults-treatment standard
- PMID: 37418237
- PMCID: PMC10794095
- DOI: 10.1093/ndt/gfad146
IgA nephropathy in adults-treatment standard
Abstract
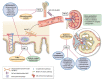
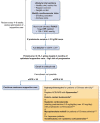
Immunoglobulin A nephropathy (IgAN) is the most common primary form of glomerular disease worldwide and carries a high lifetime risk of kidney failure. The underlying pathogenesis of IgAN has been characterized to a sub-molecular level; immune complexes containing specific O-glycoforms of IgA1 are central. Kidney biopsy remains the gold-standard diagnostic test for IgAN and histological features (i.e. MEST-C score) have also been shown to independently predict outcome. Proteinuria and blood pressure are the main modifiable risk factors for disease progression. No IgAN-specific biomarker has yet been validated for diagnosis, prognosis or tracking response to therapy. There has been a recent resurgence of investigation into IgAN treatments. Optimized supportive care with lifestyle interventions and non-immunomodulatory drugs remains the backbone of IgAN management. The menu of available reno-protective medications is rapidly expanding beyond blockade of the renin-angiotensin-aldosterone system to include sodium-glucose cotransporter 2 and endothelin type A receptor antagonism. Systemic immunosuppression can further improve kidney outcomes, although recent randomized controlled trials have raised concerns regarding infectious and metabolic toxicity from systemic corticosteroids. Studies evaluating more refined approaches to immunomodulation in IgAN are ongoing: drugs targeting the mucosal immune compartment, B-cell promoting cytokines and the complement cascade are particularly promising. We review the current standards of treatment and discuss novel developments in pathophysiology, diagnosis, outcome prediction and management of IgAN.
Keywords: IgA nephropathy; diagnosis; pathophysiology; prognosis; treatment.
© The Author(s) 2023. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
J.B. reports research grants from Argenx, Calliditas Therapeutics, Chinook Therapeutics, Galapagos, GlaxoSmithKline, Novartis and Travere Therapeutics; and is medical and/or scientific advisor to Alnylam Pharmaceuticals, Argenx, Astellas Pharma, BioCryst Pharmaceuticals, Calliditas Therapeutics, Chinook Therapeutics, Dimerix, Galapagos, Novartis, Omeros, Travere Therapeutics, UCB, Vera Therapeutics and Visterra. M.M.O’S. is a medical and/or scientific advisor to Chinook Therapeutics and Vera Therapeutics. PJG is a medical and/or scientific advisor to Calliditas Therapeutics.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

