Quantitative Fundus Autofluorescence in Systemic Chloroquine/Hydroxychloroquine Therapy: One Year Follow-Up
- PMID: 37418250
- PMCID: PMC10337803
- DOI: 10.1167/tvst.12.7.8
Quantitative Fundus Autofluorescence in Systemic Chloroquine/Hydroxychloroquine Therapy: One Year Follow-Up
Abstract
Purpose: Systemic chloroquine/hydroxychloroquine (CQ/HCQ) can cause severe ocular side effects including bull's eye maculopathy (BEM). Recently, we reported higher quantitative autofluorescence (QAF) levels in patients with CQ/HCQ intake. Here, QAF in patients taking CQ/HCQ in a 1-year follow-up is reported.
Methods: Fifty-eight patients currently or previously treated with CQ/HCQ (cumulative doses 94-2435 g) and 32 age- and sex-matched healthy subjects underwent multimodal retinal imaging (infrared, red free, fundus autofluorescence [FAF], QAF [488 nm], and spectral-domain optical coherence tomography (SD-OCT). For analysis, custom written FIJI plugins were used for image processing, multimodal image stacks assembling, and QAF calculation.
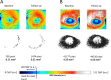
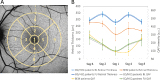
Results: Thirty patients (28 without BEM and 2 with BEM, age range = 25-69 years) were followed up (370 ± 63 days). QAF values in patients taking CQ/HCQ showed a significant increase between baseline and follow-up examination: 282.0 ± 67.9 to 297.7 ± 70.0 (QAF a.u.), P = 0.002. An increase up to 10% was observed in the superior macular hemisphere. Eight individuals (including 1 patient with BEM) had a pronounced QAF increase of up to 25%. Compared to healthy controls, QAF levels in patients taking CQ/HCQ were significantly increased (P = 0.04).
Conclusions: Our study confirms our previous finding of increased QAF in patients taking CQ/HCQ with a further significant QAF increase from baseline to follow-up. Whether pronounced QAF increase might predispose for rapid progression toward structural changes and BEM development is currently investigated in ongoing studies.
Translational relevance: In addition to standard screening tools during systemic CQ/HCQ treatment, QAF imaging might be useful in CQ/HCQ monitoring and could serve as a screening tool in the future.
Conflict of interest statement
Disclosure:
Figures




References
-
- Melles RB, Marmor MF.. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014; 132(12): 1453–1460. - PubMed
-
- Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF.. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016; 123(6): 1386–1394. - PubMed
-
- Tsang AC, Ahmadi S, Hamilton J, et al. .. The diagnostic utility of multifocal electroretinography in detecting chloroquine and hydroxychloroquine retinal toxicity. Am J Ophthalmol. 2019; 206: 132–139. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

