Association of High-Dose Erythropoietin With Circulating Biomarkers and Neurodevelopmental Outcomes Among Neonates With Hypoxic Ischemic Encephalopathy: A Secondary Analysis of the HEAL Randomized Clinical Trial
- PMID: 37418263
- PMCID: PMC10329214
- DOI: 10.1001/jamanetworkopen.2023.22131
Association of High-Dose Erythropoietin With Circulating Biomarkers and Neurodevelopmental Outcomes Among Neonates With Hypoxic Ischemic Encephalopathy: A Secondary Analysis of the HEAL Randomized Clinical Trial
Abstract
Importance: The ability to predict neurodevelopmental impairment (NDI) for infants diagnosed with hypoxic ischemic encephalopathy (HIE) is important for parental guidance and clinical treatment as well as for stratification of patients for future neurotherapeutic studies.
Objectives: To examine the effect of erythropoietin on plasma inflammatory mediators in infants with moderate or severe HIE and to develop a panel of circulating biomarkers that improves the projection of 2-year NDI over and above the clinical data available at the time of birth.
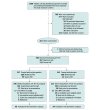
Design, setting, and participants: This study is a preplanned secondary analysis of prospectively collected data from infants enrolled in the High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL) Trial, which tested the efficacy of erythropoietin as an adjunctive neuroprotective therapy to therapeutic hypothermia. The study was conducted at 17 academic sites comprising 23 neonatal intensive care units in the United States between January 25, 2017, and October 9, 2019, with follow-up through October 2022. Overall, 500 infants born at 36 weeks' gestation or later with moderate or severe HIE were included.
Intervention: Erythropoietin treatment 1000 U/kg/dose on days 1, 2, 3, 4 and 7.
Main outcomes and measures: Plasma erythropoietin was measured in 444 infants (89%) within 24 hours after birth. A subset of 180 infants who had plasma samples available at baseline (day 0/1), day 2, and day 4 after birth and either died or had 2-year Bayley Scales of Infant Development III assessments completed were included in the biomarker analysis.
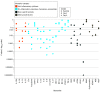
Results: The 180 infants included in this substudy had a mean (SD) gestational age of 39.1 (1.5) weeks, and 83 (46%) were female. Infants who received erythropoietin had increased concentrations of erythropoietin at day 2 and day 4 compared with baseline. Erythropoietin treatment did not alter concentrations of other measured biomarkers (eg, difference in interleukin [IL] 6 between groups on day 4: -1.3 pg/mL; 95% CI, -4.8 to 2.0 pg/mL). After adjusting for multiple comparisons, we identified 6 plasma biomarkers (C5a, interleukin [IL] 6, and neuron-specific enolase at baseline; IL-8, tau, and ubiquitin carboxy-terminal hydrolase-L1 at day 4) that significantly improved estimations of death or NDI at 2 years compared with clinical data alone. However, the improvement was only modest, increasing the AUC from 0.73 (95% CI, 0.70-0.75) to 0.79 (95% CI, 0.77-0.81; P = .01), corresponding to a 16% (95% CI, 5%-44%) increase in correct classification of participant risk of death or NDI at 2 years.
Conclusions and relevance: In this study, erythropoietin treatment did not reduce biomarkers of neuroinflammation or brain injury in infants with HIE. Circulating biomarkers modestly improved estimation of 2-year outcomes.
Trial registration: ClinicalTrials.gov Identifier: NCT02811263.
Conflict of interest statement
Figures

References
-
- Thayyil S, Pant S, Montaldo P, et al. ; HELIX consortium . Hypothermia for moderate or severe neonatal encephalopathy in low-income and middle-income countries (HELIX): a randomised controlled trial in India, Sri Lanka, and Bangladesh. Lancet Glob Health. 2021;9(9):e1273-e1285. doi: 10.1016/S2214-109X(21)00264-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

