Pathogen and human NDPK-proteins promote AML cell survival via monocyte NLRP3-inflammasome activation
- PMID: 37418424
- PMCID: PMC10328239
- DOI: 10.1371/journal.pone.0288162
Pathogen and human NDPK-proteins promote AML cell survival via monocyte NLRP3-inflammasome activation
Abstract
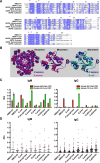
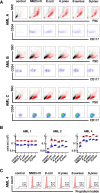
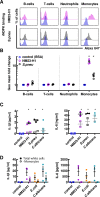
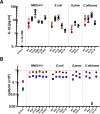
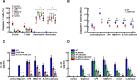
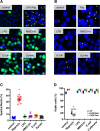
A history of infection has been linked with increased risk of acute myeloid leukaemia (AML) and related myelodysplastic syndromes (MDS). Furthermore, AML and MDS patients suffer frequent infections because of disease-related impaired immunity. However, the role of infections in the development and progression of AML and MDS remains poorly understood. We and others previously demonstrated that the human nucleoside diphosphate kinase (NDPK) NM23-H1 protein promotes AML blast cell survival by inducing secretion of IL-1β from accessory cells. NDPKs are an evolutionary highly conserved protein family and pathogenic bacteria secrete NDPKs that regulate virulence and host-pathogen interactions. Here, we demonstrate the presence of IgM antibodies against a broad range of pathogen NDPKs and more selective IgG antibody activity against pathogen NDPKs in the blood of AML patients and normal donors, demonstrating that in vivo exposure to NDPKs likely occurs. We also show that pathogen derived NDPK-proteins faithfully mimic the catalytically independent pro-survival activity of NM23-H1 against primary AML cells. Flow cytometry identified that pathogen and human NDPKs selectively bind to monocytes in peripheral blood. We therefore used vitamin D3 differentiated monocytes from wild type and genetically modified THP1 cells as a model to demonstrate that NDPK-mediated IL-1β secretion by monocytes is NLRP3-inflammasome and caspase 1 dependent, but independent of TLR4 signaling. Monocyte stimulation by NDPKs also resulted in activation of NF-κB and IRF pathways but did not include the formation of pyroptosomes or result in pyroptotic cell death which are pivotal features of canonical NLRP3 inflammasome activation. In the context of the growing importance of the NLRP3 inflammasome and IL-1β in AML and MDS, our findings now implicate pathogen NDPKs in the pathogenesis of these diseases.
Copyright: © 2023 Trova et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Kristinsson SY, Bjorkholm M, Hultcrantz M, Derolf AR, Landgren O, Goldin LR. Chronic immune stimulation might act as a trigger for the development of acute myeloid leukemia or myelodysplastic syndromes. J Clin Oncol. 2011;29(21):2897–903. Epub 2011/06/22. doi: 10.1200/JCO.2011.34.8540 ; PubMed Central PMCID: PMC3138717. - DOI - PMC - PubMed
-
- Hamalainen S, Kuittinen T, Matinlauri I, Nousiainen T, Koivula I, Jantunen E. Neutropenic fever and severe sepsis in adult acute myeloid leukemia (AML) patients receiving intensive chemotherapy: Causes and consequences. Leuk Lymphoma. 2008;49(3):495–501. Epub 2008/02/26. doi: 10.1080/10428190701809172 . - DOI - PubMed
-
- Halpern AB, Culakova E, Walter RB, Lyman GH. Association of Risk Factors, Mortality, and Care Costs of Adults With Acute Myeloid Leukemia With Admission to the Intensive Care Unit. JAMA Oncol. 2017;3(3):374–81. Epub 2016/11/11. doi: 10.1001/jamaoncol.2016.4858 ; PubMed Central PMCID: PMC5344736. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

