Altered growth and death in dilution-based viral predation assays
- PMID: 37418487
- PMCID: PMC10328242
- DOI: 10.1371/journal.pone.0288114
Altered growth and death in dilution-based viral predation assays
Abstract
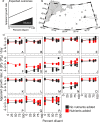
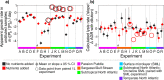
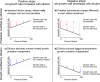
Viral lysis of phytoplankton is one of the most common forms of death on Earth. Building on an assay used extensively to assess rates of phytoplankton loss to predation by grazers, lysis rates are increasingly quantified through dilution-based techniques. In this approach, dilution of viruses and hosts are expected to reduce infection rates and thus increase host net growth rates (i.e., accumulation rates). The difference between diluted and undiluted host growth rates is interpreted as a measurable proxy for the rate of viral lytic death. These assays are usually conducted in volumes ≥ 1 L. To increase throughput, we implemented a miniaturized, high-throughput, high-replication, flow cytometric microplate dilution assay to measure viral lysis in environmental samples sourced from a suburban pond and the North Atlantic Ocean. The most notable outcome we observed was a decline in phytoplankton densities that was exacerbated by dilution, instead of the increased growth rates expected from lowered virus-phytoplankton encounters. We sought to explain this counterintuitive outcome using theoretical, environmental, and experimental analyses. Our study shows that, while die-offs could be partly explained by a 'plate effect' due to small incubation volumes and cells adhering to walls, the declines in phytoplankton densities are not volume-dependent. Rather, they are driven by many density- and physiology-dependent effects of dilution on predation pressure, nutrient limitation, and growth, all of which violate the original assumptions of dilution assays. As these effects are volume-independent, these processes likely occur in all dilution assays that our analyses show to be remarkably sensitive to dilution-altered phytoplankton growth and insensitive to actual predation pressure. Incorporating altered growth as well as predation, we present a logical framework that categorizes locations by the relative dominance of these mechanisms, with general applicability to dilution-based assays.
Copyright: © 2023 Knowles et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Latitudinal variation in virus-induced mortality of phytoplankton across the North Atlantic Ocean.ISME J. 2016 Feb;10(2):500-13. doi: 10.1038/ismej.2015.130. Epub 2015 Aug 11. ISME J. 2016. PMID: 26262815 Free PMC article.
-
The Effect of Strain Level Diversity on Robust Inference of Virus-Induced Mortality of Phytoplankton.Front Microbiol. 2018 Sep 3;9:1850. doi: 10.3389/fmicb.2018.01850. eCollection 2018. Front Microbiol. 2018. PMID: 30233501 Free PMC article.
-
Contrasting community versus population-based estimates of grazing and virus-induced mortality of phytoplankton.Microb Ecol. 2012 Jul;64(1):25-38. doi: 10.1007/s00248-012-0019-9. Epub 2012 Feb 11. Microb Ecol. 2012. PMID: 22327271
-
Differential fitness returns in relation to spatial position in groups.Biol Rev Camb Philos Soc. 1994 May;69(2):187-206. doi: 10.1111/j.1469-185x.1994.tb01505.x. Biol Rev Camb Philos Soc. 1994. PMID: 8054444 Review.
-
Comparative ecology of North Atlantic shores: do differences in players matter for process?Ecology. 2008 Nov;89(11 Suppl):S3-23. doi: 10.1890/07-1155.1. Ecology. 2008. PMID: 19097481 Review.
References
-
- Thingstad TF. Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol Oceanogr. 2000;45: 1320–1328.
-
- Record NR, Talmy D, Våge S. Quantifying Tradeoffs for Marine Viruses. Frontiers in Marine Science. 2016. p. 251.
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

