Dehydration-enhanced ion-pore interactions dominate anion transport and selectivity in nanochannels
- PMID: 37418527
- PMCID: PMC10328398
- DOI: 10.1126/sciadv.adf8412
Dehydration-enhanced ion-pore interactions dominate anion transport and selectivity in nanochannels
Abstract
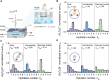
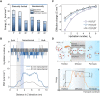
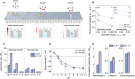
State-of-the-art ion-selective membranes with ultrahigh precision are of significance for water desalination and energy conservation, but their development is limited by the lack of understanding of the mechanisms of ion transport at the subnanometer scale. Herein, we investigate transport of three typical anions (F-, Cl-, and Br-) under confinement using in situ liquid time-of-flight secondary ion mass spectrometry in combination with transition-state theory. The operando analysis reveals that dehydration and related ion-pore interactions govern anion-selective transport. For strongly hydrated ions [(H2O)nF- and (H2O)nCl-], dehydration enhances ion effective charge and thus the electrostatic interactions with membrane, observed as an increase in decomposed energy from electrostatics, leading to more hindered transport. Contrarily, weakly hydrated ions [(H2O)nBr-] have greater permeability as they allow an intact hydration structure during transport due to their smaller size and the most right-skewed hydration distribution. Our work demonstrates that precisely regulating ion dehydration to maximize the difference in ion-pore interactions could enable the development of ideal ion-selective membranes.
Figures

Similar articles
-
Response of Ionic Hydration Structure and Selective Transport Behavior to Aqueous Solution Chemistry during Nanofiltration.Environ Sci Technol. 2024 Jul 2;58(26):11791-11801. doi: 10.1021/acs.est.4c01783. Epub 2024 Jun 13. Environ Sci Technol. 2024. PMID: 38871647
-
Steric Hindrance-Induced Dehydration Promotes Cation Selectivity in Trans-Subnanochannel Transport.ACS Nano. 2023 Jul 11;17(13):12629-12640. doi: 10.1021/acsnano.3c03028. Epub 2023 Jun 23. ACS Nano. 2023. PMID: 37350330
-
Intrapore energy barriers govern ion transport and selectivity of desalination membranes.Sci Adv. 2020 Nov 25;6(48):eabd9045. doi: 10.1126/sciadv.abd9045. Print 2020 Nov. Sci Adv. 2020. PMID: 33239305 Free PMC article.
-
Ion-Ion Selectivity of Synthetic Membranes with Confined Nanostructures.ACS Nano. 2024 Aug 20;18(33):21633-21650. doi: 10.1021/acsnano.4c00540. Epub 2024 Aug 8. ACS Nano. 2024. PMID: 39114876 Review.
-
Applying Transition-State Theory to Explore Transport and Selectivity in Salt-Rejecting Membranes: A Critical Review.Environ Sci Technol. 2022 Jun 21;56(12):7467-7483. doi: 10.1021/acs.est.2c00912. Epub 2022 May 13. Environ Sci Technol. 2022. PMID: 35549171 Review.
Cited by
-
Precision ion separation via self-assembled channels.Nat Commun. 2024 Apr 11;15(1):3160. doi: 10.1038/s41467-024-47083-0. Nat Commun. 2024. PMID: 38605042 Free PMC article.
-
Nanoconfinement Effects in Electrocatalysis and Photocatalysis.Small. 2025 Apr;21(13):e2411184. doi: 10.1002/smll.202411184. Epub 2025 Feb 24. Small. 2025. PMID: 39989153 Free PMC article. Review.
-
How to Compare the Ion Selectivity of Smart Nanopores/Membranes.Research (Wash D C). 2024 Oct 25;7:0506. doi: 10.34133/research.0506. eCollection 2024. Research (Wash D C). 2024. PMID: 40771577 Free PMC article.
-
Electrostatic-induced ion-confined partitioning in graphene nanolaminate membrane for breaking anion-cation co-transport to enhance desalination.Nat Commun. 2024 May 21;15(1):4324. doi: 10.1038/s41467-024-48681-8. Nat Commun. 2024. PMID: 38773152 Free PMC article.
-
Role of Ion Dehydration in Ion-Ion Selectivity of Dense Membranes.Environ Sci Technol. 2025 Sep 2;59(34):17997-18009. doi: 10.1021/acs.est.5c04303. Epub 2025 Aug 19. Environ Sci Technol. 2025. PMID: 40830835 Free PMC article. Review.
References
-
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas, A. M. Mayes, Science and technology for water purification in the coming decades. Nature 452, 301–310 (2008). - PubMed
-
- K. Wang, X. Wang, B. Januszewski, Y. Liu, D. Li, R. Fu, M. Elimelech, X. Huang, Tailored design of nanofiltration membranes for water treatment based on synthesis-property-performance relationships. Chem. Soc. Rev. 51, 672–719 (2022). - PubMed
-
- L. A. Richards, A. I. Schafer, B. S. Richards, B. Corry, The importance of dehydration in determining ion transport in narrow pores. Small 8, 1701–1709 (2012). - PubMed
-
- C. Lu, C. Hu, C. L. Ritt, X. Hua, J. Sun, H. Xia, Y. Liu, D. W. Li, B. Ma, M. Elimelech, J. Qu, In situ characterization of dehydration during ion transport in polymeric nanochannels. J. Am. Chem. Soc. 143, 14242–14252 (2021). - PubMed
-
- Y. F. Zhou, J. H. Morais-Cabral, A. Kaufman, R. MacKinnon, Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature 414, 43–48 (2001). - PubMed
LinkOut - more resources
Full Text Sources

