Single-cell and spatial transcriptomics identify a macrophage population associated with skeletal muscle fibrosis
- PMID: 37418531
- PMCID: PMC10328414
- DOI: 10.1126/sciadv.add9984
Single-cell and spatial transcriptomics identify a macrophage population associated with skeletal muscle fibrosis
Abstract
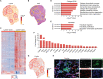
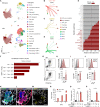
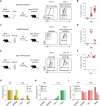
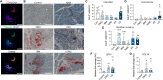
Macrophages are essential for skeletal muscle homeostasis, but how their dysregulation contributes to the development of fibrosis in muscle disease remains unclear. Here, we used single-cell transcriptomics to determine the molecular attributes of dystrophic and healthy muscle macrophages. We identified six clusters and unexpectedly found that none corresponded to traditional definitions of M1 or M2 macrophages. Rather, the predominant macrophage signature in dystrophic muscle was characterized by high expression of fibrotic factors, galectin-3 (gal-3) and osteopontin (Spp1). Spatial transcriptomics, computational inferences of intercellular communication, and in vitro assays indicated that macrophage-derived Spp1 regulates stromal progenitor differentiation. Gal-3+ macrophages were chronically activated in dystrophic muscle, and adoptive transfer assays showed that the gal-3+ phenotype was the dominant molecular program induced within the dystrophic milieu. Gal-3+ macrophages were also elevated in multiple human myopathies. These studies advance our understanding of macrophages in muscular dystrophy by defining their transcriptional programs and reveal Spp1 as a major regulator of macrophage and stromal progenitor interactions.
Figures



Update of
-
Single-cell and spatial transcriptomics identify a macrophage population associated with skeletal muscle fibrosis.bioRxiv [Preprint]. 2023 Apr 18:2023.04.18.537253. doi: 10.1101/2023.04.18.537253. bioRxiv. 2023. Update in: Sci Adv. 2023 Jul 7;9(27):eadd9984. doi: 10.1126/sciadv.add9984. PMID: 37131694 Free PMC article. Updated. Preprint.
References
-
- Okabe Y., Molecular control of the identity of tissue-resident macrophages. Int. Immunol. 30, 485–491 (2018). - PubMed
-
- Saclier M., Yacoub-Youssef H., Mackey A. L., Arnold L., Ardjoune H., Magnan M., Sailhan F., Chelly J., Pavlath G. K., Mounier R., Kjaer M., Chazaud B., Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 31, 384–396 (2013). - PubMed
MeSH terms
Substances
Grants and funding
- R01 AI168063/AI/NIAID NIH HHS/United States
- P30 AR075047/AR/NIAMS NIH HHS/United States
- R35 GM143019/GM/NIGMS NIH HHS/United States
- P50 AR052646/AR/NIAMS NIH HHS/United States
- R01 NS120060/NS/NINDS NIH HHS/United States
- R01 AR078340/AR/NIAMS NIH HHS/United States
- T32 AI060573/AI/NIAID NIH HHS/United States
- R01 GM143536/GM/NIGMS NIH HHS/United States
- UL1 TR001414/TR/NCATS NIH HHS/United States
- U54 AR052646/AR/NIAMS NIH HHS/United States
- R01 AR055686/AR/NIAMS NIH HHS/United States
- KL2 TR001416/TR/NCATS NIH HHS/United States
- P30 CA062203/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

