The interplay between the DNA damage response and ectonucleotidases modulates tumor response to therapy
- PMID: 37418547
- PMCID: PMC10394739
- DOI: 10.1126/sciimmunol.abq3015
The interplay between the DNA damage response and ectonucleotidases modulates tumor response to therapy
Abstract
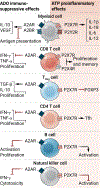
The extracellular nucleoside adenosine reduces tissue inflammation and is generated by irreversible dephosphorylation of adenosine monophosphate (AMP) mediated by the ectonucleotidase CD73. The pro-inflammatory nucleotides adenosine triphosphate, nicotinamide adenine dinucleotide, and cyclic guanosine -monophosphate-AMP (cGAMP), which are produced in the tumor microenvironment (TME) during therapy-induced immunogenic cell death and activation of innate immune signaling, can be converted into AMP by ectonucleotidases CD39, CD38, and CD203a/ENPP1. Thus, ectonucleotidases shape the TME by converting immune-activating signals into an immunosuppressive one. Ectonucleotidases also hinder the ability of therapies including radiation therapy, which enhance the release of pro-inflammatory nucleotides in the extracellular milieu, to induce immune-mediated tumor rejection. Here, we review the immunosuppressive effects of adenosine and the role of different ectonucleotidases in modulating antitumor immune responses. We discuss emerging opportunities to target adenosine generation and/or its ability to signal via adenosine receptors expressed by immune and cancer cells in the context of combination immunotherapy and radiotherapy.
Conflict of interest statement
Author contributions
All authors wrote the manuscript.
Competing interests
SD has received compensation for consultant/advisory services from Lytix Biopharma, Johnson & Johnson Enterprise Innovation Inc., EMD Serono, Ono Pharmaceutical, and Genentech, and research support from Lytix Biopharma and Boehringer-Ingelheim for unrelated projects.
JS owns stock of Surface Oncology and has received compensation for consultant/advisory services from Surface Oncology and Domain Therapeutics, and research support from Surface Oncology and Domain Therapeutics for unrelated projects. EG receives research support from Arcus Biosciences, Inc. for the PANTHER trial.
The authors declare that they have no competing interests related to this work.
Figures



References
-
- Sharma P, Allison JP, The future of immune checkpoint therapy. Science 348, 56–61 (2015). - PubMed
-
- Charpentier M, Spada S, Van Nest SJ, Demaria S, Radiation therapy-induced remodeling of the tumor immune microenvironment. Semin Cancer Biol, (2022). - PubMed
-
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L, Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 15, 1170–1178 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

