NPRA promotes fatty acid metabolism and proliferation of gastric cancer cells by binding to PPARα
- PMID: 37418841
- PMCID: PMC10345484
- DOI: 10.1016/j.tranon.2023.101734
NPRA promotes fatty acid metabolism and proliferation of gastric cancer cells by binding to PPARα
Abstract
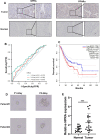
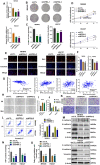
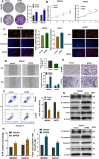
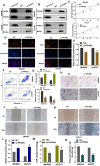
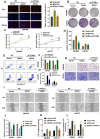
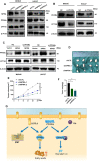
Among cancers, gastric cancer (GC) ranks third globally in morbidity and mortality, particularly in East Asia. Natriuretic peptide receptor A (NPRA), a receptor for guanylate cyclase, plays important roles in regulating water and sodium balance. Recent studies have suggested that NPRA is involved in tumorigenesis, but its role in GC development remains unclear. Herein, we showed that the expression level of NPRA was positively correlated with gastric tumor size and clinical stage. Patients with high NPRA expression had a lower five-year survival rate than those with low expression, and NPRA was identified as an independent predictor of GC prognosis. NPRA knockdown suppressed GC cell proliferation, migration and invasion. NPRA overexpression enhanced cell malignant behavior. Immunohistochemistry of collected tumor samples showed that tumors with high NPRA expression had higher peroxisome proliferator-activated receptor α (PPARα) levels. In vivo and in vitro studies showed that NPRA promotes fatty acid oxidation and tumor cell metastasis. Co-IP showed that NPRA binds to PPARα and prevents PPARα degradation. PPARα upregulation under NPRA protection activates arnitine palmitoyl transferase 1B (CPT1B) to promote fatty acid oxidation. In this study, new mechanisms by which NPRA promotes the development of GC and new regulatory mechanisms of PPARα were identified.
Keywords: CPT1B; EMT; FAO; NPRA; PPARα.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors have no conflict of interest.
Figures

References
-
- Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. - PubMed
-
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. - PubMed
-
- Lee C.K., et al. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 2019;363(6427):644–649. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

