The antioxidant l-Ergothioneine prevents cystine lithiasis in the Slc7a9-/- mouse model of cystinuria
- PMID: 37418888
- PMCID: PMC10359938
- DOI: 10.1016/j.redox.2023.102801
The antioxidant l-Ergothioneine prevents cystine lithiasis in the Slc7a9-/- mouse model of cystinuria
Abstract
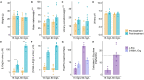
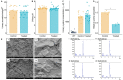
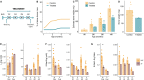
The high recurrence rate of cystine lithiasis observed in cystinuria patients highlights the need for new therapeutic options to address this chronic disease. There is growing evidence of an antioxidant defect in cystinuria, which has led to test antioxidant molecules as new therapeutic approaches. In this study, the antioxidant l-Ergothioneine was evaluated, at two different doses, as a preventive and long-term treatment for cystinuria in the Slc7a9-/- mouse model. l-Ergothioneine treatments decreased the rate of stone formation by more than 60% and delayed its onset in those mice that still developed calculi. Although there were no differences in metabolic parameters or urinary cystine concentration between control and treated mice, cystine solubility was increased by 50% in the urines of treated mice. We also demonstrate that l-Ergothioneine needs to be internalized by its transporter OCTN1 (Slc22a4) to be effective, as when administrated to the double mutant Slc7a9-/-Slc22a4-/- mouse model, no effect on the lithiasis phenotype was observed. In kidneys, we detected a decrease in GSH levels and an impairment of maximal mitochondrial respiratory capacity in cystinuric mice that l-Ergothioneine treatment was able to restore. Thus, l-Ergothioneine administration prevented cystine lithiasis in the Slc7a9-/- mouse model by increasing urinary cystine solubility and recovered renal GSH metabolism and mitochondrial function. These results support the need for clinical trials to test l-Ergothioneine as a new treatment for cystinuria.
Keywords: Antioxidant; Cystine lithiasis; Cystinuria; Oxidative stress; Treatment; l-Ergothioneine.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Virginia Nunes’ group received support funded by AVEROA SAS.
Figures


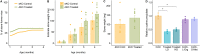
References
-
- Streeper N.M., Wertheim M.L., Nakada S.Y., Penniston K.L. Cystine stone formers have impaired health-related quality of life compared with noncystine stone formers: a case-referent study piloting the Wisconsin stone quality of life questionnaire among patients with cystine stones. J. Endourol. 2022;31(1):48–53. doi: 10.1089/end.2016.0564. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

