Open-Label Trial of Amikacin Liposome Inhalation Suspension in Mycobacterium abscessus Lung Disease
- PMID: 37419144
- PMCID: PMC10645596
- DOI: 10.1016/j.chest.2023.05.036
Open-Label Trial of Amikacin Liposome Inhalation Suspension in Mycobacterium abscessus Lung Disease
Abstract
Background: Mycobacterium abscessus is the second most common nontuberculous mycobacterium respiratory pathogen and shows in vitro resistance to nearly all oral antimicrobials. M abscessus treatment success is low in the presence of macrolide resistance.
Research question: Does treatment with amikacin liposome inhalation suspension (ALIS) improve culture conversion in patients with M abscessus pulmonary disease who are treatment naive or who have treatment-refractory disease?
Study design and methods: In an open-label protocol, patients were given ALIS (590 mg) added to background multidrug therapy for 12 months. The primary outcome was sputum culture conversion defined as three consecutive monthly sputum cultures showing negative results. The secondary end point included development of amikacin resistance.
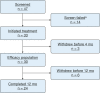
Results: Of 33 patients (36 isolates) who started ALIS with a mean age of 64 years (range, 14-81 years), 24 patients (73%) were female, 10 patients (30%) had cystic fibrosis, and nine patients (27%) had cavitary disease. Three patients (9%) could not be evaluated for the microbiologic end point because of early withdrawal. All pretreatment isolates were amikacin susceptible and only six isolates (17%) were macrolide susceptible. Eleven patients (33%) were given parenteral antibiotics. Twelve patients (40%) received clofazimine with or without azithromycin as companion therapy. Fifteen patients (50%) with evaluable longitudinal microbiologic data demonstrated culture conversion, and 10 patients (67%) sustained conversion through month 12. Six of the 33 patients (18%) demonstrated mutational amikacin resistance. All were patients using clofazimine or clofazimine plus azithromycin as companion medication(s). Few serious adverse events occurred for ALIS users; however, reduction of dosing to three times weekly was common (52%).
Interpretation: In a cohort of patients primarily with macrolide-resistant M abscessus, one-half of the patients using ALIS showed sputum culture conversion to negative findings. The emergence of mutational amikacin resistance was not uncommon and occurred with the use of clofazimine monotherapy.
Trial registry: ClinicalTrials.gov; No.: NCT03038178; URL: www.
Clinicaltrials: gov.
Keywords: Mycobacterium abscessus; amikacin liposome inhalation suspension; nontuberculous mycobacteria lung disease.
Copyright © 2023 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Financial/Nonfinancial Disclosures The authors have reported to CHEST the following: D. E. G. received support as a consultant from Insmed, Inc., AN2 Therapeutics, and Paratek Pharmaceuticals and received research support from Insmed, Inc. J. V. P. received support as part of advisory boards for Insmed, Inc., AN2 Therapeutics, and Paratek Pharmaceuticals and received research support from Insmed, Inc., AN2 Therapeutics, Paratek Pharmaceuticals, Electromed, Inc., Hillrom, and Zambon. R. J. W. received research support from Insmed, Inc. K. L. W. received support as a consultant from Insmed, Inc., AN2 Therapeutics, Paratek Pharmaceuticals, RedHill Biopharma, Renovion, Inc., and Spero Therapeutics and received research support from Insmed, Inc., AN2 Therapeutics, Paratek Pharmaceuticals, RedHill Biopharma, and Renovion, Inc. None declared (S. A. R. S., B. A. B.-E., A. E. B., P. E. S., C. F., L. S.).
Figures
References
-
- Wagner D.L.M., Cooray S., Ringshausen F.C., Morimoto K., Koh W.J., Thomson R. In: Nontuberculous Mycobacterial Disease. Respiratory Medicine. Griffith D.E., editor. Humana Press, Cham; 2019. Global epidemiology of NTM disease (except Northern America) pp. 163–260.
-
- Tortoli E., Kohl T.A., Brown-Elliott B.A., et al. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii and designation of Mycobacteriumabscessus subsp. massiliense comb. nov. Int J Syst Evol Microbiol. 2016;66(11):4471–4479. - PubMed
-
- Griffith D.E., Brown-Elliott B.A., Benwill J.L., Wallace R.J., Jr., Mycobacterium abscessus “Pleased to meet you, hope you guess my name . . . .”. Ann Am Thorac Soc. 2015;12(3):436–439. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical