Different Mouse Models of Nemaline Myopathy Harboring Acta1 Mutations Display Differing Abnormalities Related to Mitochondrial Biology
- PMID: 37419385
- PMCID: PMC10548277
- DOI: 10.1016/j.ajpath.2023.06.008
Different Mouse Models of Nemaline Myopathy Harboring Acta1 Mutations Display Differing Abnormalities Related to Mitochondrial Biology
Abstract
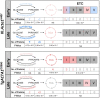
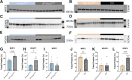
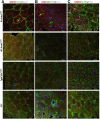
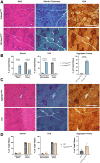
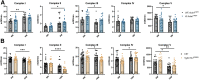
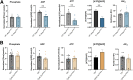
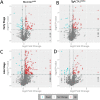
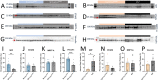
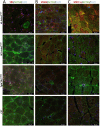
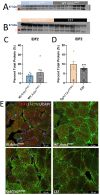
ACTA1 encodes skeletal muscle-specific α-actin, which polymerizes to form the thin filament of the sarcomere. Mutations in ACTA1 are responsible for approximately 30% of nemaline myopathy (NM) cases. Previous studies of weakness in NM have focused on muscle structure and contractility, but genetic issues alone do not explain the phenotypic heterogeneity observed in patients with NM or NM mouse models. To identify additional biological processes related to NM phenotypic severity, proteomic analysis was performed using muscle protein isolates from wild-type mice in comparison to moderately affected knock-in (KI) Acta1H40Y and the minimally affected transgenic (Tg) ACTA1D286G NM mice. This analysis revealed abnormalities in mitochondrial function and stress-related pathways in both mouse models, supporting an in-depth assessment of mitochondrial biology. Interestingly, evaluating each model in comparison to its wild-type counterpart identified different degrees of mitochondrial abnormality that correlated well with the phenotypic severity of the mouse model. Muscle histology, mitochondrial respiration, electron transport chain function, and mitochondrial transmembrane potential were all normal or minimally affected in the TgACTA1D286G mouse model. In contrast, the more severely affected KI.Acta1H40Y mice displayed significant abnormalities in relation to muscle histology, mitochondrial respirometry, ATP, ADP, and phosphate content, and mitochondrial transmembrane potential. These findings suggest that abnormal energy metabolism is related to symptomatic severity in NM and may constitute a contributor to phenotypic variability and a novel treatment target.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
ACTA1 H40Y mutant iPSC-derived skeletal myocytes display mitochondrial defects in an in vitro model of nemaline myopathy.Exp Cell Res. 2023 Mar 15;424(2):113507. doi: 10.1016/j.yexcr.2023.113507. Epub 2023 Feb 14. Exp Cell Res. 2023. PMID: 36796746 Free PMC article.
-
Combined MRI and ³¹P-MRS investigations of the ACTA1(H40Y) mouse model of nemaline myopathy show impaired muscle function and altered energy metabolism.PLoS One. 2013 Apr 16;8(4):e61517. doi: 10.1371/journal.pone.0061517. Print 2013. PLoS One. 2013. PMID: 23613869 Free PMC article.
-
Multimodal MRI and (31)P-MRS investigations of the ACTA1(Asp286Gly) mouse model of nemaline myopathy provide evidence of impaired in vivo muscle function, altered muscle structure and disturbed energy metabolism.PLoS One. 2013 Aug 20;8(8):e72294. doi: 10.1371/journal.pone.0072294. eCollection 2013. PLoS One. 2013. PMID: 23977274 Free PMC article.
-
Clinical and Histologic Findings in ACTA1-Related Nemaline Myopathy: Case Series and Review of the Literature.Pediatr Neurol. 2017 Oct;75:11-16. doi: 10.1016/j.pediatrneurol.2017.04.002. Epub 2017 Apr 7. Pediatr Neurol. 2017. PMID: 28780987 Review.
-
Skeletal muscle α-actin diseases (actinopathies): pathology and mechanisms.Acta Neuropathol. 2013 Jan;125(1):19-32. doi: 10.1007/s00401-012-1019-z. Epub 2012 Jul 24. Acta Neuropathol. 2013. PMID: 22825594 Review.
Cited by
-
An Update on Reported Variants in the Skeletal Muscle α-Actin (ACTA1) Gene.Hum Mutat. 2024 Oct 28;2024:6496088. doi: 10.1155/2024/6496088. eCollection 2024. Hum Mutat. 2024. PMID: 40225930 Free PMC article. Review.
-
Actin Polymerization Defects Induce Mitochondrial Dysfunction in Cellular Models of Nemaline Myopathies.Antioxidants (Basel). 2023 Nov 21;12(12):2023. doi: 10.3390/antiox12122023. Antioxidants (Basel). 2023. PMID: 38136143 Free PMC article.
-
Tirasemtiv enhances submaximal muscle tension in an Acta1:p.Asp286Gly mouse model of nemaline myopathy.J Gen Physiol. 2024 Apr 1;156(4):e202313471. doi: 10.1085/jgp.202313471. Epub 2024 Feb 20. J Gen Physiol. 2024. PMID: 38376469 Free PMC article.
-
Integrated single-cell functional-proteomic profiling reveals a shift in myofibre specificity in human nemaline myopathy: A proof-of-principle study.J Physiol. 2025 May;603(10):3033-3048. doi: 10.1113/JP288363. Epub 2025 May 5. J Physiol. 2025. PMID: 40320980 Free PMC article.
-
Aberrations in Energetic Metabolism and Stress-Related Pathways Contribute to Pathophysiology in the Neb Conditional Knockout Mouse Model of Nemaline Myopathy.Am J Pathol. 2023 Oct;193(10):1528-1547. doi: 10.1016/j.ajpath.2023.06.009. Epub 2023 Jul 6. Am J Pathol. 2023. PMID: 37422147 Free PMC article.
References
-
- Nilipour Y., Nafissi S., Tjust A.E., Ravenscroft G., Hossein Nejad Nedai H., Taylor R.L., Varasteh V., Pedrosa Domellof F., Zangi M., Tonekaboni S.H., Olive M., Kiiski K., Sagath L., Davis M.R., Laing N.G., Tajsharghi H. Ryanodine receptor type 3 (RYR3) as a novel gene associated with a myopathy with nemaline bodies. Eur J Neurol. 2018;25:841–847. - PubMed
-
- Ryan M.M., Ilkovski B., Strickland C.D., Schnell C., Sanoudou D., Midgett C., Houston R., Muirhead D., Dennett X., Shield L.K., De Girolami U., Iannaccone S.T., Laing N.G., North K.N., Beggs A.H. Clinical course correlates poorly with muscle pathology in nemaline myopathy. Neurology. 2003;60:665–673. - PubMed
-
- Agrawal P.B., Strickland C.D., Midgett C., Morales A., Newburger D.E., Poulos M.A., Tomczak K.K., Ryan M.M., Iannaccone S.T., Crawford T.O., Laing N.G., Beggs A.H. Heterogeneity of nemaline myopathy cases with skeletal muscle alpha-actin gene mutations. Ann Neurol. 2004;56:86–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

