Protocol for a cluster randomised placebo-controlled trial of adjunctive ivermectin mass drug administration for malaria control on the Bijagós Archipelago of Guinea-Bissau: the MATAMAL trial
- PMID: 37419638
- PMCID: PMC10335573
- DOI: 10.1136/bmjopen-2023-072347
Protocol for a cluster randomised placebo-controlled trial of adjunctive ivermectin mass drug administration for malaria control on the Bijagós Archipelago of Guinea-Bissau: the MATAMAL trial
Abstract
Introduction: As malaria declines, innovative tools are required to further reduce transmission and achieve elimination. Mass drug administration (MDA) of artemisinin-based combination therapy (ACT) is capable of reducing malaria transmission where coverage of control interventions is already high, though the impact is short-lived. Combining ACT with ivermectin, an oral endectocide shown to reduce vector survival, may increase its impact, while also treating ivermectin-sensitive co-endemic diseases and minimising the potential impact of ACT resistance in this context.
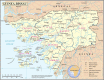
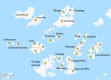
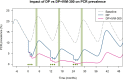
Methods and analysis: MATAMAL is a cluster-randomised placebo-controlled trial. The trial is being conducted in 24 clusters on the Bijagós Archipelago, Guinea-Bissau, where the peak prevalence of Plasmodium falciparum (Pf) parasitaemia is approximately 15%. Clusters have been randomly allocated to receive MDA with dihydroartemisinin-piperaquine and either ivermectin or placebo. The primary objective is to determine whether the addition of ivermectin MDA is more effective than dihydroartemisinin-piperaquine MDA alone in reducing the prevalence of P. falciparum parasitaemia, measured during peak transmission season after 2 years of seasonal MDA. Secondary objectives include assessing prevalence after 1 year of MDA; malaria incidence monitored through active and passive surveillance; age-adjusted prevalence of serological markers indicating exposure to P. falciparum and anopheline mosquitoes; vector parous rates, species composition, population density and sporozoite rates; prevalence of vector pyrethroid resistance; prevalence of artemisinin resistance in P. falciparum using genomic markers; ivermectin's impact on co-endemic diseases; coverage estimates; and the safety of combined MDA.
Ethics and dissemination: The trial has been approved by the London School of Hygiene and Tropical Medicine's Ethics Committee (UK) (19156) and the Comite Nacional de Eticas de Saude (Guinea-Bissau) (084/CNES/INASA/2020). Results will be disseminated in peer-reviewed publications and in discussion with the Bissau-Guinean Ministry of Public Health and participating communities.
Trial registration number: NCT04844905.
Keywords: clinical trials; epidemiologic studies; guinea-bissau; ivermectin; malaria; mass drug administration.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: JGL declares he is founder/CEO of Arctech Innovation, a company which aims to design mosquito lures and malaria diagnostics.
Figures


References
-
- Barnes KG, Weedall GD, Ndula M, et al. . Genomic footprints of selective sweeps from metabolic resistance to Pyrethroids in African malaria vectors are driven by scale up of insecticide-based vector control. PLOS Genet 2017;13:e1006539. 10.1371/journal.pgen.1006539 Available: https://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen.1... - DOI - PMC - PubMed
-
- Global Malaria Programme . Malaria policy advisory committee meeting report. Geneva, 2016. Available: https://www.who.int/publications/i/item/WHO-HTM-GMP-MPAC-2016.3
-
- Bousema T, Okell L, Felger I, et al. . Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol 2014;12:833–40. 10.1038/nrmicro3364 Available: https://pubmed.ncbi.nlm.nih.gov/25329408/ - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
