Restrictive versus Liberal Rate of Extracorporeal Volume Removal Evaluation in Acute Kidney Injury (RELIEVE-AKI): a pilot clinical trial protocol
- PMID: 37419639
- PMCID: PMC10335418
- DOI: 10.1136/bmjopen-2023-075960
Restrictive versus Liberal Rate of Extracorporeal Volume Removal Evaluation in Acute Kidney Injury (RELIEVE-AKI): a pilot clinical trial protocol
Abstract
Introduction: Observational studies have linked slower and faster net ultrafiltration (UFNET) rates during kidney replacement therapy (KRT) with mortality in critically ill patients with acute kidney injury (AKI) and fluid overload. To inform the design of a larger randomised trial of patient-centered outcomes, we conduct a feasibility study to examine restrictive and liberal approaches to UFNET during continuous KRT (CKRT).
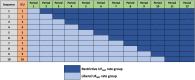
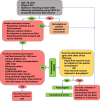
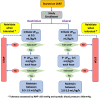
Methods and analysis: This study is an investigator-initiated, unblinded, 2-arm, comparative-effectiveness, stepped-wedged, cluster randomised trial among 112 critically ill patients with AKI treated with CKRT in 10 intensive care units (ICUs) across 2 hospital systems. In the first 6 months, all ICUs started with a liberal UFNET rate strategy. Thereafter, one ICU is randomised to the restrictive UFNET rate strategy every 2 months. In the liberal group, the UFNET rate is maintained between 2.0 and 5.0 mL/kg/hour; in the restrictive group, the UFNET rate is maintained between 0.5 and 1.5 mL/kg/hour. The three coprimary feasibility outcomes are (1) between-group separation in mean delivered UFNET rates; (2) protocol adherence; and (3) patient recruitment rate. Secondary outcomes include daily and cumulative fluid balance, KRT and mechanical ventilation duration, organ failure-free days, ICU and hospital length of stay, hospital mortality and KRT dependence at hospital discharge. Safety endpoints include haemodynamics, electrolyte imbalance, CKRT circuit issues, organ dysfunction related to fluid overload, secondary infections and thrombotic and haematological complications.
Ethics and dissemination: The University of Pittsburgh Human Research Protection Office approved the study, and an independent Data and Safety Monitoring Board monitors the study. A grant from the United States National Institute of Diabetes and Digestive and Kidney Diseases sponsors the study. The trial results will be submitted for publication in peer-reviewed journals and presented at scientific conferences.
Trial registration number: This trial has been prospectively registered with clinicaltrials.gov (NCT05306964). Protocol version identifier and date: 1.5; 13 June 2023.
Keywords: Acute renal failure; Adult intensive & critical care; Clinical Trial; Dialysis.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RM received research grants from NIDDK, consulting fees from Baxter, AM Pharma, Bioporto and La Jolla unrelated to this study; DTH received grants from NIH. KK received research grants NIDDK and from Philips Research North America and Google, speaker honorarium from Nikkiso Critical Care Medical Supplies (Shanghai) and consulting fees to Mayo Clinic and from Baxter; PMP received consulting fees and advisory committee fees from Durect, Health-Span Dx and Novartis; served on a Data and Safety Monitoring Board for Baxter; served as a member of an endpoint adjudication committee for GE Healthcare; and CCH, MR and NN has nothing to disclose.
Figures
Similar articles
-
Fluid Overload and Precision Net Ultrafiltration in Critically Ill Patients.Cardiorenal Med. 2023;13(1):9-18. doi: 10.1159/000527390. Epub 2022 Oct 6. Cardiorenal Med. 2023. PMID: 36202071 Free PMC article. Review.
-
Fluid balance neutralization secured by hemodynamic monitoring versus protocolized standard of care in critically ill patients requiring continuous renal replacement therapy: study protocol of the GO NEUTRAL randomized controlled trial.Trials. 2022 Sep 22;23(1):798. doi: 10.1186/s13063-022-06735-6. Trials. 2022. PMID: 36138465 Free PMC article.
-
Association between Net Ultrafiltration Rate and Renal Recovery among Critically Ill Adults with Acute Kidney Injury Receiving Continuous Renal Replacement Therapy: An Observational Cohort Study.Blood Purif. 2022;51(5):397-409. doi: 10.1159/000517281. Epub 2021 Jul 21. Blood Purif. 2022. PMID: 34289471
-
Forced fluid removal versus usual care in intensive care patients with high-risk acute kidney injury and severe fluid overload (FFAKI): study protocol for a randomised controlled pilot trial.Trials. 2017 Apr 24;18(1):189. doi: 10.1186/s13063-017-1935-2. Trials. 2017. PMID: 28438182 Free PMC article. Clinical Trial.
-
Ultrafiltration in critically ill patients treated with kidney replacement therapy.Nat Rev Nephrol. 2021 Apr;17(4):262-276. doi: 10.1038/s41581-020-00358-3. Epub 2020 Nov 11. Nat Rev Nephrol. 2021. PMID: 33177700 Review.
Cited by
-
Response Letter to: Correspondence regarding the article by Murugan et al on Precision net ultrafiltration dosing in continuous kidney replacement therapy: a practical approach.Intensive Care Med Exp. 2024 Feb 20;12(1):17. doi: 10.1186/s40635-023-00588-2. Intensive Care Med Exp. 2024. PMID: 38376597 Free PMC article. No abstract available.
-
Behind the scenes: Key lessons learned from the RELIEVE-AKI clinical trial.J Crit Care. 2024 Oct;83:154845. doi: 10.1016/j.jcrc.2024.154845. Epub 2024 Jun 15. J Crit Care. 2024. PMID: 38879964 Review.
-
Precision net ultrafiltration dosing in continuous kidney replacement therapy: a practical approach.Intensive Care Med Exp. 2023 Nov 28;11(1):83. doi: 10.1186/s40635-023-00566-8. Intensive Care Med Exp. 2023. PMID: 38015332 Free PMC article. No abstract available.
References
-
- Kidney Disease Improving Global Outcomes (KDIGO) Workgroup . Clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
