Porous microneedle patch with sustained delivery of extracellular vesicles mitigates severe spinal cord injury
- PMID: 37419902
- PMCID: PMC10328956
- DOI: 10.1038/s41467-023-39745-2
Porous microneedle patch with sustained delivery of extracellular vesicles mitigates severe spinal cord injury
Erratum in
-
Author Correction: Porous microneedle patch with sustained delivery of extracellular vesicles mitigates severe spinal cord injury.Nat Commun. 2023 Aug 1;14(1):4603. doi: 10.1038/s41467-023-40368-w. Nat Commun. 2023. PMID: 37528087 Free PMC article. No abstract available.
Abstract
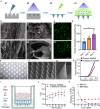
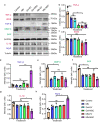
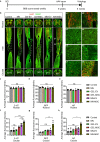
The transplantation of mesenchymal stem cells-derived secretome, particularly extracellular vesicles is a promising therapy to suppress spinal cord injury-triggered neuroinflammation. However, efficient delivery of extracellular vesicles to the injured spinal cord, with minimal damage, remains a challenge. Here we present a device for the delivery of extracellular vesicles to treat spinal cord injury. We show that the device incorporating mesenchymal stem cells and porous microneedles enables the delivery of extracellular vesicles. We demonstrate that topical application to the spinal cord lesion beneath the spinal dura, does not damage the lesion. We evaluate the efficacy of our device in a contusive spinal cord injury model and find that it reduces the cavity and scar tissue formation, promotes angiogenesis, and improves survival of nearby tissues and axons. Importantly, the sustained delivery of extracellular vesicles for at least 7 days results in significant functional recovery. Thus, our device provides an efficient and sustained extracellular vesicles delivery platform for spinal cord injury treatment.
© 2023. The Author(s).
Conflict of interest statement
Zhejiang University has filed a patent application (application number: 2022108442387, applied) related to this work, with X.W., A.F., and B.G. listed as inventors. X.W. is a scientific co-founder of WeQure AI Ltd. All the other authors declare no competing interests.
Figures




References
-
- Ahuja CS, et al. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017;3:1–21. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

