Plakoglobin is a mechanoresponsive regulator of naive pluripotency
- PMID: 37419903
- PMCID: PMC10329048
- DOI: 10.1038/s41467-023-39515-0
Plakoglobin is a mechanoresponsive regulator of naive pluripotency
Abstract
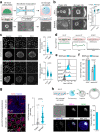
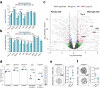
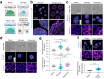
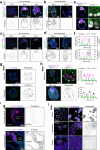
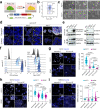
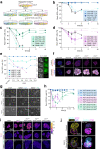
Biomechanical cues are instrumental in guiding embryonic development and cell differentiation. Understanding how these physical stimuli translate into transcriptional programs will provide insight into mechanisms underlying mammalian pre-implantation development. Here, we explore this type of regulation by exerting microenvironmental control over mouse embryonic stem cells. Microfluidic encapsulation of mouse embryonic stem cells in agarose microgels stabilizes the naive pluripotency network and specifically induces expression of Plakoglobin (Jup), a vertebrate homolog of β-catenin. Overexpression of Plakoglobin is sufficient to fully re-establish the naive pluripotency gene regulatory network under metastable pluripotency conditions, as confirmed by single-cell transcriptome profiling. Finally, we find that, in the epiblast, Plakoglobin was exclusively expressed at the blastocyst stage in human and mouse embryos - further strengthening the link between Plakoglobin and naive pluripotency in vivo. Our work reveals Plakoglobin as a mechanosensitive regulator of naive pluripotency and provides a paradigm to interrogate the effects of volumetric confinement on cell-fate transitions.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Lineage-Specific Profiling Delineates the Emergence and Progression of Naive Pluripotency in Mammalian Embryogenesis.Dev Cell. 2015 Nov 9;35(3):366-82. doi: 10.1016/j.devcel.2015.10.011. Dev Cell. 2015. PMID: 26555056 Free PMC article.
-
Ectopic γ-catenin expression partially mimics the effects of stabilized β-catenin on embryonic stem cell differentiation.PLoS One. 2013 May 27;8(5):e65320. doi: 10.1371/journal.pone.0065320. Print 2013. PLoS One. 2013. PMID: 23724138 Free PMC article.
-
Loss of the Otx2-Binding Site in the Nanog Promoter Affects the Integrity of Embryonic Stem Cell Subtypes and Specification of Inner Cell Mass-Derived Epiblast.Cell Rep. 2016 Jun 21;15(12):2651-64. doi: 10.1016/j.celrep.2016.05.041. Epub 2016 Jun 9. Cell Rep. 2016. PMID: 27292645
-
Stepwise pluripotency transitions in mouse stem cells.EMBO Rep. 2022 Sep 5;23(9):e55010. doi: 10.15252/embr.202255010. Epub 2022 Jul 29. EMBO Rep. 2022. PMID: 35903955 Free PMC article. Review.
-
Capturing Pluripotency and Beyond.Cells. 2021 Dec 16;10(12):3558. doi: 10.3390/cells10123558. Cells. 2021. PMID: 34944066 Free PMC article. Review.
Cited by
-
Marmoset and human trophoblast stem cells differ in signaling requirements and recapitulate divergent modes of trophoblast invasion.Cell Stem Cell. 2024 Oct 3;31(10):1427-1446.e8. doi: 10.1016/j.stem.2024.09.004. Epub 2024 Sep 24. Cell Stem Cell. 2024. PMID: 39321797 Free PMC article.
-
Fluorogenic, Subsingle-Turnover Monitoring of Enzymatic Reactions Involving NAD(P)H Provides a Generalized Platform for Directed Ultrahigh-Throughput Evolution of Biocatalysts in Microdroplets.J Am Chem Soc. 2025 Apr 2;147(13):10903-10915. doi: 10.1021/jacs.4c11804. Epub 2025 Mar 24. J Am Chem Soc. 2025. PMID: 40127491 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

