Endothelial cell CD36 regulates membrane ceramide formation, exosome fatty acid transfer and circulating fatty acid levels
- PMID: 37419919
- PMCID: PMC10329018
- DOI: 10.1038/s41467-023-39752-3
Endothelial cell CD36 regulates membrane ceramide formation, exosome fatty acid transfer and circulating fatty acid levels
Abstract
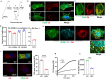
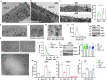
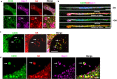
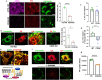
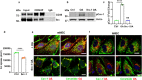
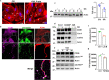
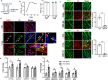
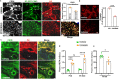
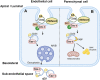
Endothelial cell (EC) CD36 controls tissue fatty acid (FA) uptake. Here we examine how ECs transfer FAs. FA interaction with apical membrane CD36 induces Src phosphorylation of caveolin-1 tyrosine-14 (Cav-1Y14) and ceramide generation in caveolae. Ensuing fission of caveolae yields vesicles containing FAs, CD36 and ceramide that are secreted basolaterally as small (80-100 nm) exosome-like extracellular vesicles (sEVs). We visualize in transwells EC transfer of FAs in sEVs to underlying myotubes. In mice with EC-expression of the exosome marker emeraldGFP-CD63, muscle fibers accumulate circulating FAs in emGFP-labeled puncta. The FA-sEV pathway is mapped through its suppression by CD36 depletion, blocking actin-remodeling, Src inhibition, Cav-1Y14 mutation, and neutral sphingomyelinase 2 inhibition. Suppression of sEV formation in mice reduces muscle FA uptake, raises circulating FAs, which remain in blood vessels, and lowers glucose, mimicking prominent Cd36-/- mice phenotypes. The findings show that FA uptake influences membrane ceramide, endocytosis, and EC communication with parenchymal cells.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Endothelial cell CD36 optimizes tissue fatty acid uptake.J Clin Invest. 2018 Oct 1;128(10):4329-4342. doi: 10.1172/JCI99315. Epub 2018 Jul 26. J Clin Invest. 2018. PMID: 30047927 Free PMC article.
-
CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis.Nat Commun. 2020 Sep 21;11(1):4765. doi: 10.1038/s41467-020-18565-8. Nat Commun. 2020. PMID: 32958780 Free PMC article.
-
Peroxisome proliferator-activated receptor-γ in capillary endothelia promotes fatty acid uptake by heart during long-term fasting.J Am Heart Assoc. 2013 Jan 18;2(1):e004861. doi: 10.1161/JAHA.112.004861. J Am Heart Assoc. 2013. PMID: 23525438 Free PMC article.
-
Cellular fatty acid uptake: a pathway under construction.Trends Endocrinol Metab. 2009 Mar;20(2):72-7. doi: 10.1016/j.tem.2008.11.001. Epub 2009 Jan 29. Trends Endocrinol Metab. 2009. PMID: 19185504 Free PMC article. Review.
-
Fatty Acid Transport and Signaling: Mechanisms and Physiological Implications.Annu Rev Physiol. 2023 Feb 10;85:317-337. doi: 10.1146/annurev-physiol-032122-030352. Epub 2022 Nov 8. Annu Rev Physiol. 2023. PMID: 36347219 Review.
Cited by
-
Celebrating the 1945 JNCI pioneering contribution to antiangiogenic therapy for cancer.J Natl Cancer Inst. 2024 Nov 1;116(11):1715-1720. doi: 10.1093/jnci/djae181. J Natl Cancer Inst. 2024. PMID: 39178374 Free PMC article. No abstract available.
-
VPS28 regulates triglyceride synthesis via ubiquitination in bovine mammary epithelial cells.Sci Rep. 2024 Dec 28;14(1):31310. doi: 10.1038/s41598-024-82774-0. Sci Rep. 2024. PMID: 39732879 Free PMC article.
-
NRAC controls CD36-mediated fatty acid uptake in adipocytes and lipid clearance in vivo.EMBO J. 2025 Aug 1. doi: 10.1038/s44318-025-00520-2. Online ahead of print. EMBO J. 2025. PMID: 40750702
-
m6A-modified exosome-derived circHIF1α binding to KH domain of IGF2BP3 mediates DNA damage and arrests G1/S transition phase to resists bacterial infection in bacteremia.J Nanobiotechnology. 2024 Oct 24;22(1):654. doi: 10.1186/s12951-024-02932-4. J Nanobiotechnology. 2024. PMID: 39443946 Free PMC article.
-
The role of exosomes in immunopathology and potential therapeutic implications.Cell Mol Immunol. 2025 Jul 14. doi: 10.1038/s41423-025-01323-5. Online ahead of print. Cell Mol Immunol. 2025. PMID: 40659888 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

