Fast-spiking parvalbumin-positive interneurons in brain physiology and Alzheimer's disease
- PMID: 37419975
- PMCID: PMC11041664
- DOI: 10.1038/s41380-023-02168-y
Fast-spiking parvalbumin-positive interneurons in brain physiology and Alzheimer's disease
Abstract
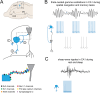
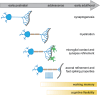
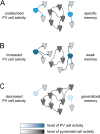
Fast-spiking parvalbumin (PV) interneurons are inhibitory interneurons with unique morphological and functional properties that allow them to precisely control local circuitry, brain networks and memory processing. Since the discovery in 1987 that PV is expressed in a subset of fast-spiking GABAergic inhibitory neurons, our knowledge of the complex molecular and physiological properties of these cells has been expanding. In this review, we highlight the specific properties of PV neurons that allow them to fire at high frequency and with high reliability, enabling them to control network oscillations and shape the encoding, consolidation and retrieval of memories. We next discuss multiple studies reporting PV neuron impairment as a critical step in neuronal network dysfunction and cognitive decline in mouse models of Alzheimer's disease (AD). Finally, we propose potential mechanisms underlying PV neuron dysfunction in AD and we argue that early changes in PV neuron activity could be a causal step in AD-associated network and memory impairment and a significant contributor to disease pathogenesis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Parvalbumin Interneuron Dysfunction in Neurological Disorders: Focus on Epilepsy and Alzheimer's Disease.Int J Mol Sci. 2024 May 19;25(10):5549. doi: 10.3390/ijms25105549. Int J Mol Sci. 2024. PMID: 38791587 Free PMC article. Review.
-
Fast-spiking parvalbumin-positive interneurons: new perspectives of treatment and future challenges in dementia.Mol Psychiatry. 2025 Feb;30(2):693-704. doi: 10.1038/s41380-024-02756-6. Epub 2024 Dec 18. Mol Psychiatry. 2025. PMID: 39695324 Review.
-
Early alterations in hippocampal perisomatic GABAergic synapses and network oscillations in a mouse model of Alzheimer's disease amyloidosis.PLoS One. 2019 Jan 15;14(1):e0209228. doi: 10.1371/journal.pone.0209228. eCollection 2019. PLoS One. 2019. PMID: 30645585 Free PMC article.
-
Inhibitory Parvalbumin Basket Cell Activity is Selectively Reduced during Hippocampal Sharp Wave Ripples in a Mouse Model of Familial Alzheimer's Disease.J Neurosci. 2020 Jun 24;40(26):5116-5136. doi: 10.1523/JNEUROSCI.0425-20.2020. Epub 2020 May 21. J Neurosci. 2020. PMID: 32439703 Free PMC article.
-
Interneurons. Fast-spiking, parvalbumin⁺ GABAergic interneurons: from cellular design to microcircuit function.Science. 2014 Aug 1;345(6196):1255263. doi: 10.1126/science.1255263. Epub 2014 Jul 31. Science. 2014. PMID: 25082707 Review.
Cited by
-
Resilience to Alzheimer's disease associates with alterations in perineuronal nets.Alzheimers Dement. 2025 Feb;21(2):e14504. doi: 10.1002/alz.14504. Epub 2024 Dec 31. Alzheimers Dement. 2025. PMID: 39737731 Free PMC article.
-
How dopamine tunes parvalbumin interneurons in the hippocampus: new experimental observations in Alzheimer's disease.Neural Regen Res. 2025 May 1;20(5):1405-1406. doi: 10.4103/NRR.NRR-D-24-00322. Epub 2024 Jun 26. Neural Regen Res. 2025. PMID: 39075905 Free PMC article. No abstract available.
-
Functional network disruption in cognitively unimpaired autosomal dominant Alzheimer's disease: a magnetoencephalography study.Brain Commun. 2024 Nov 25;6(6):fcae423. doi: 10.1093/braincomms/fcae423. eCollection 2024. Brain Commun. 2024. PMID: 39713236 Free PMC article.
-
Quantification and correlation of amyloid-β plaque load, glial activation, GABAergic interneuron numbers, and cognitive decline in the young TgF344-AD rat model of Alzheimer's disease.Front Aging Neurosci. 2025 Feb 12;17:1542229. doi: 10.3389/fnagi.2025.1542229. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40013092 Free PMC article.
-
Upregulated inwardly rectifying K + current-mediated hypoactivity of parvalbumin interneurons underlies autism-like deficits in Bod1-deficient mice.J Biomed Res. 2025 Mar 31;39(4):417-429. doi: 10.7555/JBR.38.20240394. J Biomed Res. 2025. PMID: 40164568 Free PMC article.
References
-
- Hu H, Gan J, Jonas P. Fast-spiking, parvalbumin + GABAergic interneurons: From cellular design to microcircuit function. Science. 2014;345:1255263. - PubMed
-
- Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron. 2008;57:536–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

