Amphetamine disrupts dopamine axon growth in adolescence by a sex-specific mechanism in mice
- PMID: 37419977
- PMCID: PMC10329029
- DOI: 10.1038/s41467-023-39665-1
Amphetamine disrupts dopamine axon growth in adolescence by a sex-specific mechanism in mice
Abstract
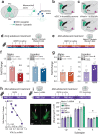
Initiating drug use during adolescence increases the risk of developing addiction or other psychopathologies later in life, with long-term outcomes varying according to sex and exact timing of use. The cellular and molecular underpinnings explaining this differential sensitivity to detrimental drug effects remain unexplained. The Netrin-1/DCC guidance cue system segregates cortical and limbic dopamine pathways in adolescence. Here we show that amphetamine, by dysregulating Netrin-1/DCC signaling, triggers ectopic growth of mesolimbic dopamine axons to the prefrontal cortex, only in early-adolescent male mice, underlying a male-specific vulnerability to enduring cognitive deficits. In adolescent females, compensatory changes in Netrin-1 protect against the deleterious consequences of amphetamine on dopamine connectivity and cognitive outcomes. Netrin-1/DCC signaling functions as a molecular switch which can be differentially regulated by the same drug experience as function of an individual's sex and adolescent age, and lead to divergent long-term outcomes associated with vulnerable or resilient phenotypes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
DCC-related developmental effects of abused- versus therapeutic-like amphetamine doses in adolescence.Addict Biol. 2020 Jul;25(4):e12791. doi: 10.1111/adb.12791. Epub 2019 Jun 13. Addict Biol. 2020. PMID: 31192517 Free PMC article.
-
DCC Receptors Drive Prefrontal Cortex Maturation by Determining Dopamine Axon Targeting in Adolescence.Biol Psychiatry. 2018 Jan 15;83(2):181-192. doi: 10.1016/j.biopsych.2017.06.009. Epub 2017 Jun 16. Biol Psychiatry. 2018. PMID: 28720317 Free PMC article.
-
The Netrin-1/DCC guidance system: dopamine pathway maturation and psychiatric disorders emerging in adolescence.Mol Psychiatry. 2020 Feb;25(2):297-307. doi: 10.1038/s41380-019-0561-7. Epub 2019 Oct 28. Mol Psychiatry. 2020. PMID: 31659271 Free PMC article. Review.
-
Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a DCC-dependent manner.Neuropsychopharmacology. 2015 Mar 13;40(5):1101-12. doi: 10.1038/npp.2014.287. Neuropsychopharmacology. 2015. PMID: 25336209 Free PMC article.
-
Making Dopamine Connections in Adolescence.Trends Neurosci. 2017 Dec;40(12):709-719. doi: 10.1016/j.tins.2017.09.004. Epub 2017 Oct 9. Trends Neurosci. 2017. PMID: 29032842 Free PMC article. Review.
Cited by
-
The scheduling of adolescence with Netrin-1 and UNC5C.Elife. 2024 Jul 26;12:RP88261. doi: 10.7554/eLife.88261. Elife. 2024. PMID: 39056276 Free PMC article.
-
Prenatal Delta-9-Tetrahydrocannabinol Exposure Induces Transcriptional Alterations in Dopaminergic System with Associated Electrophysiological Dysregulation in the Prefrontal Cortex of Adolescent Rats.Cells. 2025 Jun 14;14(12):904. doi: 10.3390/cells14120904. Cells. 2025. PMID: 40558531 Free PMC article.
-
Prefrontal cortex development and its implications in mental illness.Neuropsychopharmacology. 2025 Jul 3. doi: 10.1038/s41386-025-02154-8. Online ahead of print. Neuropsychopharmacology. 2025. PMID: 40603750 Review.
-
Timing of Methamphetamine Exposure during Adolescence Differentially Influences Parvalbumin and Perineuronal Net Immunoreactivity in the Medial Prefrontal Cortex of Female, but Not Male, Rats.Dev Neurosci. 2025;47(1):27-39. doi: 10.1159/000538608. Epub 2024 Mar 28. Dev Neurosci. 2025. PMID: 38547851 Free PMC article.
-
We're not in Kansas anymore: ectopic dopaminergic terminals as an explanation for the positive symptoms in psychiatric pathology.J Psychiatry Neurosci. 2023 Feb 21;48(1):E74-E77. doi: 10.1503/jpn.230015. Print 2023 Jan-Feb. J Psychiatry Neurosci. 2023. PMID: 36810305 Free PMC article. No abstract available.
References
-
- Schneider, M. Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res.354, 99–106 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

