Proton pump inhibitor use: systematic review of global trends and practices
- PMID: 37420019
- PMCID: PMC10427555
- DOI: 10.1007/s00228-023-03534-z
Proton pump inhibitor use: systematic review of global trends and practices
Abstract
Purpose: Proton pump inhibitors (PPIs) reduce acid secretion in the stomach and rank as one of the most widely used acid-suppressing medicines globally. While PPIs are safe in the short-term, emerging evidence shows risks associated with long-term use. Current evidence on global PPI use is scarce. This systematic review aims to evaluate global PPI use in the general population.
Methods: Ovid MEDLINE, Embase, and International Pharmaceutical Abstracts were systematically searched from inception to 31 March 2023 to identify observational studies on oral PPI use among individuals aged ≥ 18 years. PPI use was classified by demographics and medication factors (dose, duration, and PPI types). The absolute numbers of PPI users for each subcategory were summed and expressed as a percentage.
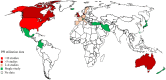
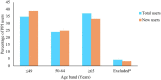
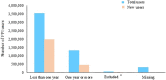
Results: The search identified data from 28 million PPI users in 23 countries from 65 articles. This review indicated that nearly one-quarter of adults use a PPI. Of those using PPIs, 63% were less than 65 years. 56% of PPI users were female, and "White" ethnicities accounted for 75% of users. Nearly two-thirds of users were on high doses (≥ defined daily dose (DDD)), 25% of users continued PPIs for > 1 year, and 28% of these continued for > 3 years.
Conclusion: Given the widespread use PPIs and increasing concern regarding long-term use, this review provides a catalyst to support more rational use, particularly with unnecessary prolonged continuation. Clinicians should review PPI prescriptions regularly and deprescribe when there is no appropriate ongoing indication or evidence of benefit to reduce health harm and treatment cost.
Keywords: Dose; Global use; Long term; Proton pump inhibitors; Systematic review.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Missing PPI prescriptions while overprescribing?Eur J Clin Pharmacol. 2023 Nov;79(11):1579-1581. doi: 10.1007/s00228-023-03564-7. Epub 2023 Sep 19. Eur J Clin Pharmacol. 2023. PMID: 37725121 Free PMC article. No abstract available.
Similar articles
-
Deprescribing versus continuation of chronic proton pump inhibitor use in adults.Cochrane Database Syst Rev. 2017 Mar 16;3(3):CD011969. doi: 10.1002/14651858.CD011969.pub2. Cochrane Database Syst Rev. 2017. PMID: 28301676 Free PMC article.
-
Proton pump inhibitors for functional dyspepsia.Cochrane Database Syst Rev. 2017 Nov 21;11(11):CD011194. doi: 10.1002/14651858.CD011194.pub3. Cochrane Database Syst Rev. 2017. PMID: 29161458 Free PMC article.
-
Proton pump inhibitors for functional dyspepsia.Cochrane Database Syst Rev. 2017 Mar 8;3(3):CD011194. doi: 10.1002/14651858.CD011194.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2017 Nov 21;11:CD011194. doi: 10.1002/14651858.CD011194.pub3. PMID: 28271513 Free PMC article. Updated.
-
Risk of thromboembolism in patients with COVID-19 who are using hormonal contraception.Cochrane Database Syst Rev. 2023 Jan 9;1(1):CD014908. doi: 10.1002/14651858.CD014908.pub2. Cochrane Database Syst Rev. 2023. Update in: Cochrane Database Syst Rev. 2023 May 15;5:CD014908. doi: 10.1002/14651858.CD014908.pub3. PMID: 36622724 Free PMC article. Updated.
-
Smoking cessation medicines and e-cigarettes: a systematic review, network meta-analysis and cost-effectiveness analysis.Health Technol Assess. 2021 Oct;25(59):1-224. doi: 10.3310/hta25590. Health Technol Assess. 2021. PMID: 34668482
Cited by
-
Patterns of outpatient proton‒pump inhibitors use among older adults in a duplicative health system: comparing public and private prescribing.BMC Health Serv Res. 2025 Jan 6;25(1):30. doi: 10.1186/s12913-024-12033-5. BMC Health Serv Res. 2025. PMID: 39762802 Free PMC article.
-
Practices and knowledge of community pharmacists towards the use of proton pump inhibitors: a cross-sectional study in Jordan.BMJ Open. 2025 Feb 8;15(2):e085589. doi: 10.1136/bmjopen-2024-085589. BMJ Open. 2025. PMID: 39922594 Free PMC article.
-
DepRescribing inapprOpriate Proton Pump InhibiTors (DROPIT): study protocol of a cluster-randomised controlled trial in Swiss primary care.BMJ Open. 2025 Jan 20;15(1):e094495. doi: 10.1136/bmjopen-2024-094495. BMJ Open. 2025. PMID: 39832992 Free PMC article.
-
Proton-pump inhibitors increase C. difficile infection risk by altering pH rather than by affecting the gut microbiome based on a bioreactor model.Gut Microbes. 2025 Dec;17(1):2519697. doi: 10.1080/19490976.2025.2519697. Epub 2025 Jun 16. Gut Microbes. 2025. PMID: 40524314 Free PMC article.
-
Risk of stroke associated with proton pump inhibitor use among individuals with and without pre-existing cardiovascular diseases: a systematic review and meta-analysis.J Cardiothorac Surg. 2025 Jan 29;20(1):107. doi: 10.1186/s13019-024-03161-4. J Cardiothorac Surg. 2025. PMID: 39881328 Free PMC article.
References
-
- New Zealand Formulary (NZF) (2021) New Zealand Formulary (NZF), NZF v[114]. Available from: www.nzf.org.nz. Accessed 1 Dec 2021
-
- National Institute for Health and Care Excellence (NICE) (2019) Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management Clinical guideline (CG184). Available from: https://www.nice.org.uk/guidance/cg184/chapter/Appendix-A-Dosage-informa.... Accessed on 1 Dec 2021 - PubMed
-
- Hu X, You X, Sun X, Lv J, Wu Y, Liu Q, et al. Off label use of proton pump inhibitors and economic burden in Chinese population: a retrospective analysis using claims database. Value in Health. 2018;21:S82–S83. doi: 10.1016/j.jval.2018.04.560. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

