CXCL5 suppression recovers neovascularization and accelerates wound healing in diabetes mellitus
- PMID: 37420254
- PMCID: PMC10329364
- DOI: 10.1186/s12933-023-01900-w
CXCL5 suppression recovers neovascularization and accelerates wound healing in diabetes mellitus
Erratum in
-
Correction: CXCL5 suppression recovers neovascularization and accelerates wound healing in diabetes mellitus.Cardiovasc Diabetol. 2023 Aug 1;22(1):196. doi: 10.1186/s12933-023-01929-x. Cardiovasc Diabetol. 2023. PMID: 37528428 Free PMC article. No abstract available.
Abstract
Background: Higher chemokine C-X-C motif ligand 5 (CXCL5) level was observed in type 2 diabetes mellitus (DM) patients; however, its role in diabetic vasculopathy was not clarified. This study aimed to explore the impacts and mechanistic insights of CXCL5 in neovasculogenesis and wound healing in DM.
Methods: Endothelial progenitor cells (EPCs) and human aortic endothelial cells (HAECs) were used in vitro. Streptozotocin-induced diabetic mice and Leprdb/JNarl mice were used as type 1 and type 2 DM models. Moreover, CXCL5 knockout mice were used to generate diabetic mice. Hindlimb ischemia surgery, aortic ring assays, matrigel plug assay, and wound healing assay were conducted.
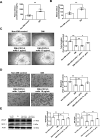
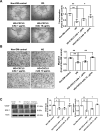
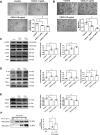
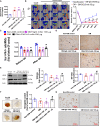
Results: CXCL5 concentrations were increased in plasma and EPCs culture medium from type 2 DM patients. CXCL5 neutralizing antibody upregulated vascular endothelial growth factor (VEGF)/stromal cell-derived factor-1 (SDF-1) and promoted cell function in EPCs from type 2 DM patients and high glucose-treated EPCs from non-DM subjects as well as HAECs. CXCL5 directly up-regulated interleukin (IL)-1β/IL-6/tumor necrosis factor-α and down-regulated VEGF/SDF-1 via ERK/p65 activation through chemokine C-X-C motif receptor 2 (CXCR2). CXCL5 neutralizing antibody recovered the blood flow after hindlimb ischemia, increased circulating EPC number, and enhanced VEGF and SDF-1 expression in ischemic muscle. CXCL5 suppression promoted neovascularization and wound healing in different diabetic animal models. The above observation could also be seen in streptozotocin-induced CXCL5 knockout diabetic mice.
Conclusions: CXCL5 suppression could improve neovascularization and wound healing through CXCR2 in DM. CXCL5 may be regarded as a potential therapeutic target for vascular complications of DM.
Keywords: CXCL5; CXCR2; Diabetes mellitus; Neovascularization; Wound healing.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

