Biallelic DDHD2 mutations in patients with adult-onset complex hereditary spastic paraplegia
- PMID: 37420318
- PMCID: PMC10502669
- DOI: 10.1002/acn3.51850
Biallelic DDHD2 mutations in patients with adult-onset complex hereditary spastic paraplegia
Abstract
Objective: Hereditary spastic paraplegias (HSPs) are a group of inherited neurodegenerative disorders characterized by slowly progressive lower limb spasticity and weakness. HSP type 54 (SPG54) is autosomal recessively inherited and caused by mutations in the DDHD2 gene. This study investigated the clinical characteristics and molecular features of DDHD2 mutations in a cohort of Taiwanese patients with HSP.
Methods: Mutational analysis of DDHD2 was performed for 242 unrelated Taiwanese patients with HSP. The clinical, neuroimaging, and genetic features of the patients with biallelic DDHD2 mutations were characterized. A cell-based study was performed to assess the effects of the DDHD2 mutations on protein expression.
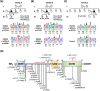
Results: SPG54 was diagnosed in three patients. Among them, two patients carried compound heterozygous DDHD2 mutations, p.[R112Q];[Y606*] and p.[R112Q];[p.D660H], and the other one was homozygous for the DDHD2 p.R112Q mutation. DDHD2 p.Y606* is a novel mutation, whereas DDHD2 p.D660H and p.R112Q have been reported in the literature. All three patients manifested adult onset complex HSP with additional cerebellar ataxia, polyneuropathy, or cognitive impairment. Brain proton magnetic resonance spectroscopy revealed an abnormal lipid peak in thalamus of all three patients. In vitro studies demonstrated that all the three DDHD2 mutations were associated with a considerably lower DDHD2 protein level.
Interpretation: SPG54 was detected in approximately 1.2% (3 of 242) of the Taiwanese HSP cohort. This study expands the known mutational spectrum of DDHD2, provides molecular evidence of the pathogenicity of the DDHD2 mutations, and underlines the importance of considering SPG54 as a potential diagnosis of adult-onset HSP.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors declared no conflict of interests.
Figures


Similar articles
-
Defining the clinical-genetic and neuroradiological features in SPG54: description of eight additional cases and nine novel DDHD2 variants.J Neurol. 2019 Nov;266(11):2657-2664. doi: 10.1007/s00415-019-09466-y. Epub 2019 Jul 13. J Neurol. 2019. PMID: 31302745
-
Mutations in CYP2U1, DDHD2 and GBA2 genes are rare causes of complicated forms of hereditary spastic paraparesis.J Neurol. 2014 Feb;261(2):373-81. doi: 10.1007/s00415-013-7206-6. Epub 2013 Dec 13. J Neurol. 2014. PMID: 24337409
-
Mutations in phospholipase DDHD2 cause autosomal recessive hereditary spastic paraplegia (SPG54).Eur J Hum Genet. 2013 Nov;21(11):1214-8. doi: 10.1038/ejhg.2013.29. Epub 2013 Mar 13. Eur J Hum Genet. 2013. PMID: 23486545 Free PMC article.
-
Clinical and genetic characterization of NIPA1 mutations in a Taiwanese cohort with hereditary spastic paraplegia.Ann Clin Transl Neurol. 2023 Mar;10(3):353-362. doi: 10.1002/acn3.51724. Epub 2023 Jan 6. Ann Clin Transl Neurol. 2023. PMID: 36607129 Free PMC article. Review.
-
Hereditary spastic paraplegia: clinico-pathologic features and emerging molecular mechanisms.Acta Neuropathol. 2013 Sep;126(3):307-28. doi: 10.1007/s00401-013-1115-8. Epub 2013 Jul 30. Acta Neuropathol. 2013. PMID: 23897027 Free PMC article. Review.
Cited by
-
DDHD2, whose mutations cause spastic paraplegia type 54, enhances lipophagy via engaging ATG8 family proteins.Cell Death Differ. 2024 Mar;31(3):348-359. doi: 10.1038/s41418-024-01261-1. Epub 2024 Feb 8. Cell Death Differ. 2024. PMID: 38332048 Free PMC article.
References
-
- Fink JK. Advances in the hereditary spastic paraplegias. Exp Neurol. 2003;184(Suppl 1):S106‐S110. - PubMed
-
- Shribman S, Reid E, Crosby AH, Houlden H, Warner TT. Hereditary spastic paraplegia: from diagnosis to emerging therapeutic approaches. Lancet Neurol. 2019;18(12):1136‐1146. - PubMed
-
- Panza E, Meyyazhagan A, Orlacchio A. Hereditary spastic paraplegia: genetic heterogeneity and common pathways. Exp Neurol. 2022;357:114203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

