Naturalistic Hyperscanning with Wearable Magnetoencephalography
- PMID: 37420622
- PMCID: PMC10304205
- DOI: 10.3390/s23125454
Naturalistic Hyperscanning with Wearable Magnetoencephalography
Abstract
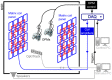
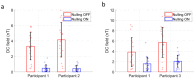
The evolution of human cognitive function is reliant on complex social interactions which form the behavioural foundation of who we are. These social capacities are subject to dramatic change in disease and injury; yet their supporting neural substrates remain poorly understood. Hyperscanning employs functional neuroimaging to simultaneously assess brain activity in two individuals and offers the best means to understand the neural basis of social interaction. However, present technologies are limited, either by poor performance (low spatial/temporal precision) or an unnatural scanning environment (claustrophobic scanners, with interactions via video). Here, we describe hyperscanning using wearable magnetoencephalography (MEG) based on optically pumped magnetometers (OPMs). We demonstrate our approach by simultaneously measuring brain activity in two subjects undertaking two separate tasks-an interactive touching task and a ball game. Despite large and unpredictable subject motion, sensorimotor brain activity was delineated clearly, and the correlation of the envelope of neuronal oscillations between the two subjects was demonstrated. Our results show that unlike existing modalities, OPM-MEG combines high-fidelity data acquisition and a naturalistic setting and thus presents significant potential to investigate neural correlates of social interaction.
Keywords: electromagnetic coil; hyperscanning; magnetic shielding; magnetoencephalography; on-scalp MEG; optically pumped magnetometer.
Conflict of interest statement
E.B. and M.J.B. are directors of Cerca Magnetics Limited, a spin-out company whose aim is to commercialise aspects of OPM-MEG technology. E.B., M.J.B., R.B., N.H. and R.H. hold founding equity in Cerca Magnetics Limited, and R.B., N.H. and R.H. sit on the scientific advisory board. M.R. is an employee of Cerca Magnetics Limited. V.S. is the founding director of QuSpin Inc., a commercial entity selling the OPM magnetometers used in this work. J.O. is an employee of QuSpin Inc. N.H, M.J.B. and R.B. declare that they have a patent pending to the UK Government Intellectual Property Office (Application No. GB2109459.4) regarding the multi-coil active magnetic shielding systems described in this work. T.M.F, M.J.B. and R.B. declare that they have a worldwide patent (WIPO Patent Application WO/2021/053356) regarding related electromagnetic coil design techniques. The remaining authors J.L., L.J.E., P.G. and P.R.M. declare no competing interest.
Figures



References
-
- Czeszumski A., Eustergerling S., Lang A., Menrath D., Gerstenberger M., Schuberth S., Schreiber F., Rendon Z.Z., König P. Hyperscanning: A Valid Method to Study Neural Inter-Brain Underpinnings of Social Interaction. Front. Hum. Neurosci. 2020;14:39. doi: 10.3389/fnhum.2020.00039. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

