Development of Optical Label-Free Biosensor Method in Detection of Listeria monocytogenes from Food
- PMID: 37420736
- PMCID: PMC10301194
- DOI: 10.3390/s23125570
Development of Optical Label-Free Biosensor Method in Detection of Listeria monocytogenes from Food
Abstract
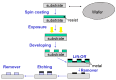
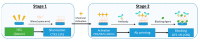
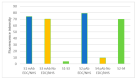
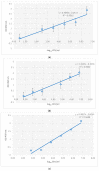
The present work describes an alternative method for detecting and identifying Listeria monocytogenes in food samples by developing a nanophotonic biosensor containing bioreceptors and optical transducers. The development of photonic sensors for the detection of pathogens in the food industry involves the implementation of procedures for selecting probes against the antigens of interest and the functionalization of the sensor surfaces on which the said bioreceptors are located. As a previous step to functionalizing the biosensor, an immobilization control of these antibodies on silicon nitride surfaces was carried out to check the effectiveness of in plane immobilization. On the one hand, it was observed that a Listeria monocytogenes-specific polyclonal antibody has a greater binding capacity to the antigen at a wide range of concentrations. A Listeria monocytogenes monoclonal antibody is more specific and has a greater binding capacity only at low concentrations. An assay for evaluating selected antibodies against particular antigens of Listeria monocytogenes bacteria was designed to determine the binding specificity of each probe using the indirect ELISA detection technique. In addition, a validation method was established against the reference method for many replicates belonging to different batches of meat-detectable samples, with a medium and pre-enrichment time that allowed optimal recovery of the target microorganism. Moreover, no cross-reactivity with other nontarget bacteria was observed. Thus, this system is a simple, highly sensitive, and accurate platform for L. monocytogenes detection.
Keywords: Listeria monocytogenes; bioreceptor; biosensor; food safety; nanophotonics; optical transducers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Antibody-aptamer functionalized fibre-optic biosensor for specific detection of Listeria monocytogenes from food.J Appl Microbiol. 2010 Sep;109(3):808-17. doi: 10.1111/j.1365-2672.2010.04709.x. J Appl Microbiol. 2010. PMID: 20337767
-
Simultaneous Detection of Escherichia coli O157:H7, Salmonella enteritidis, and Listeria monocytogenes at a Very Low Level Using Simultaneous Enrichment Broth and Multichannel SPR Biosensor.J Food Sci. 2017 Oct;82(10):2357-2363. doi: 10.1111/1750-3841.13843. Epub 2017 Aug 23. J Food Sci. 2017. PMID: 28833106
-
A sensitive impedance biosensor based on immunomagnetic separation and urease catalysis for rapid detection of Listeria monocytogenes using an immobilization-free interdigitated array microelectrode.Biosens Bioelectron. 2015 Dec 15;74:504-11. doi: 10.1016/j.bios.2015.06.007. Epub 2015 Jun 6. Biosens Bioelectron. 2015. PMID: 26176211
-
Biosensor for the detection of Listeria monocytogenes: emerging trends.Crit Rev Microbiol. 2018 Sep;44(5):590-608. doi: 10.1080/1040841X.2018.1473331. Epub 2018 May 23. Crit Rev Microbiol. 2018. PMID: 29790396 Review.
-
Fluorescence-Free Biosensor Methods in Detection of Food Pathogens with a Special Focus on Listeria monocytogenes.Biosensors (Basel). 2017 Dec 20;7(4):63. doi: 10.3390/bios7040063. Biosensors (Basel). 2017. PMID: 29261134 Free PMC article. Review.
Cited by
-
Emerging trends in nano-sensors: A new frontier in food safety and quality assurance.Heliyon. 2024 Dec 12;11(1):e41181. doi: 10.1016/j.heliyon.2024.e41181. eCollection 2025 Jan 15. Heliyon. 2024. PMID: 39807502 Free PMC article. Review.
-
A Photonic Immunosensor Detection Method for Viable and Non-Viable E. coli in Water Samples.Microorganisms. 2024 Jun 29;12(7):1328. doi: 10.3390/microorganisms12071328. Microorganisms. 2024. PMID: 39065096 Free PMC article.
References
-
- Gallagher L., Ebel E.D., Kause J.R. Draft FSIS Risk Assessment for Listeria, Ready-to-eat Meat and Poultry Products. Food Safety and Inspection Service; Washington, DC, USA: 2003.
-
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union. 2005;L338:1–26.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

