Fabrication of Carbon Nanofiber Incorporated with CuWO4 for Sensitive Electrochemical Detection of 4-Nitrotoluene in Water Samples
- PMID: 37420832
- PMCID: PMC10301614
- DOI: 10.3390/s23125668
Fabrication of Carbon Nanofiber Incorporated with CuWO4 for Sensitive Electrochemical Detection of 4-Nitrotoluene in Water Samples
Abstract
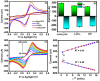
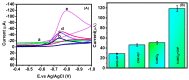
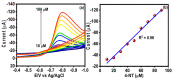
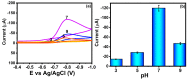
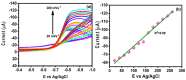
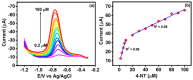
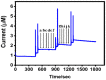
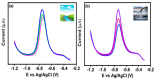
In the current work, copper tungsten oxide (CuWO4) nanoparticles are incorporated with carbon nanofiber (CNF) to form CNF/CuWO4 nanocomposite through a facile hydrothermal method. The prepared CNF/CuWO4 composite was applied to the electrochemical detection of hazardous organic pollutants of 4-nitrotoluene (4-NT). The well-defined CNF/CuWO4 nanocomposite is used as a modifier of glassy carbon electrode (GCE) to form CuWO4/CNF/GCE electrode for the detection of 4-NT. The physicochemical properties of CNF, CuWO4, and CNF/CuWO4 nanocomposite were examined by various characterization techniques, such as X-ray diffraction studies, field emission scanning electron microscopy, EDX-energy dispersive X-ray microanalysis, and high-resolution transmission electron microscopy. The electrochemical detection of 4-NT was evaluated using cyclic voltammetry (CV) the differential pulse voltammetry detection technique (DPV). The aforementioned CNF, CuWO4, and CNF/CuWO4 materials have better crystallinity with porous nature. The prepared CNF/CuWO4 nanocomposite has better electrocatalytic ability compared to other materials such as CNF, and CuWO4. The CuWO4/CNF/GCE electrode exhibited remarkable sensitivity of 7.258 μA μM-1 cm-2, a low limit of detection of 86.16 nM, and a long linear range of 0.2-100 μM. The CuWO4/CNF/GCE electrode exhibited distinguished selectivity, acceptable stability of about 90%, and well reproducibility. Meanwhile, the GCE/CNF/CuWO4 electrode has been applied to real sample analysis with better recovery results of 91.51 to 97.10%.
Keywords: 4-nitrotoluene; CNF; CuWO4; electrochemical sensor; real sample analysis.
Conflict of interest statement
The authors declare no competing interest.
Figures





Similar articles
-
Bismuth molybdate incorporated functionalized carbon nanofiber as an electrocatalytic tool for the pinpoint detection of organic pollutant in life samples.Ecotoxicol Environ Saf. 2021 Feb;209:111828. doi: 10.1016/j.ecoenv.2020.111828. Epub 2020 Dec 29. Ecotoxicol Environ Saf. 2021. PMID: 33385681
-
Electrochemical detection of nicotine at a carbon Nanofiber-Poly(amidoamine) dendrimer modified glassy carbon electrode.Chemosphere. 2022 Sep;303(Pt 1):134961. doi: 10.1016/j.chemosphere.2022.134961. Epub 2022 May 13. Chemosphere. 2022. PMID: 35577133
-
Synergistic activation of lamellar bismuth selenide anchored functionalized carbon nanofiber for detecting hazardous carbendazim in environmental water samples.Chemosphere. 2024 May;355:141744. doi: 10.1016/j.chemosphere.2024.141744. Epub 2024 Mar 22. Chemosphere. 2024. PMID: 38522669
-
Entrapment of bimetallic CoFeSe2 nanosphere on functionalized carbon nanofiber for selective and sensitive electrochemical detection of caffeic acid in wine samples.Anal Chim Acta. 2018 May 2;1006:22-32. doi: 10.1016/j.aca.2017.12.044. Epub 2018 Jan 6. Anal Chim Acta. 2018. PMID: 30016261
-
Electrochemical sensing of nicotine using CuWO4 decorated reduced graphene oxide immobilized glassy carbon electrode.Ultrason Sonochem. 2019 Jul;55:196-206. doi: 10.1016/j.ultsonch.2019.01.038. Epub 2019 Jan 29. Ultrason Sonochem. 2019. PMID: 30878204 Review.
Cited by
-
High sensitivity voltammetric sensor of 4-nitrotoluene based on nanoflake-rich boron-doped carbon nanowall electrode for water safety.Mikrochim Acta. 2025 Mar 5;192(4):208. doi: 10.1007/s00604-025-07065-5. Mikrochim Acta. 2025. PMID: 40045111
References
-
- Chakraborty U., Garg P., Bhanjana G., Kaur G., Kaushik A., Chaudhary G.R. Spherical Silver Oxide Nanoparticles for Fabrication of Electrochemical Sensor for Efficient 4-Nitrotoluene Detection and Assessment of their Antimicrobial Activity. Sci. Total Environ. 2022;808:152179. doi: 10.1016/j.scitotenv.2021.152179. - DOI - PubMed
-
- Chakraborty U., Kaur I., Chauhan A., Kaur N., Kaur G., Chaudhary G.R. Zinc oxide-Copper Sulfide Semiconductor Nano-heterostructure for Low-Level Electrochemical Detection of 4-Nitrotoluene. Electrochim. Acta. 2023;447:142160. doi: 10.1016/j.electacta.2023.142160. - DOI
-
- Easwaramoorthy D., Yu Y.C., Huang H.J. Chemiluminescence Detection of Paracetamol by a Luminol-Permanganate Based Reaction. Anal. Chim. Acta. 2001;439:95–100. doi: 10.1016/S0003-2670(01)00968-0. - DOI
-
- Sazinas R., Andersen S.Z., Li K., Saccoccio M., Krempl K., Pedersen J.B., Chorkendorff I. Towards Understanding of Electrolyte Degradation in Lithium-Mediated Non-aqueous Electrochemical Ammonia Synthesis with Gas chromatography-Mass Spectrometry. RSC Adv. 2021;11:31487–31498. doi: 10.1039/D1RA05963G. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

