Influence of electric field, blood velocity, and pharmacokinetics on electrochemotherapy efficiency
- PMID: 37421133
- PMCID: PMC10465711
- DOI: 10.1016/j.bpj.2023.07.004
Influence of electric field, blood velocity, and pharmacokinetics on electrochemotherapy efficiency
Abstract
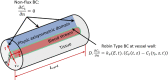
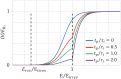
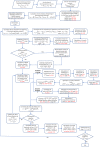
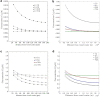
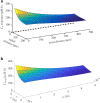
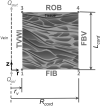
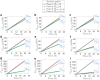
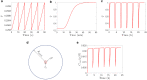
The convective delivery of chemotherapeutic drugs in cancerous tissues is directly proportional to the blood perfusion rate, which in turns can be transiently reduced by the application of high-voltage and short-duration electric pulses due to vessel vasoconstriction. However, electric pulses can also increase vessel wall and cell membrane permeabilities, boosting the extravasation and cell internalization of drug. These opposite effects, as well as possible adverse impacts on the viability of tissues and endothelial cells, suggest the importance of conducting in silico studies about the influence of physical parameters involved in electric-mediated drug transport. In the present work, the global method of approximate particular solutions for axisymmetric domains, together with two solution schemes (Gauss-Seidel iterative and linearization+successive over-relaxation), is applied for the simulation of drug transport in electroporated cancer tissues, using a continuum tumor cord approach and considering both the electropermeabilization and vasoconstriction phenomena. The developed global method of approximate particular solutions algorithm is validated with numerical and experimental results previously published, obtaining a satisfactory accuracy and convergence. Then, a parametric study about the influence of electric field magnitude and inlet blood velocity on the internalization efficacy, drug distribution uniformity, and cell-kill capacity of the treatment, as expressed by the number of internalized moles into viable cells, homogeneity of exposure to bound intracellular drug, and cell survival fraction, respectively, is analyzed for three pharmacokinetic profiles, namely one-short tri-exponential, mono-exponential, and uniform. According to numerical results, the trade-off between vasoconstriction and electropermeabilization effects and, consequently, the influence of electric field magnitude and inlet blood velocity on the assessment parameters considered here (efficacy, uniformity, and cell-kill capacity) is different for each pharmacokinetic profile deemed.
Copyright © 2023 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures











Similar articles
-
In silico study about the influence of electroporation parameters on the cellular internalization, spatial uniformity, and cytotoxic effects of chemotherapeutic drugs using the Method of Fundamental Solutions.Med Biol Eng Comput. 2024 Mar;62(3):713-749. doi: 10.1007/s11517-023-02964-2. Epub 2023 Nov 21. Med Biol Eng Comput. 2024. PMID: 37989990
-
Influence of electric pulse characteristics on the cellular internalization of chemotherapeutic drugs and cell survival fraction in electroporated and vasoconstricted cancer tissues using boundary element techniques.J Math Biol. 2023 Jul 18;87(2):31. doi: 10.1007/s00285-023-01963-z. J Math Biol. 2023. PMID: 37462802
-
Influence of electroporation parameters on the reaction and transport mechanisms in electro-chemotherapeutic treatments using Boolean modeling and the Method of Fundamental Solutions.Comput Biol Med. 2025 Feb;185:109543. doi: 10.1016/j.compbiomed.2024.109543. Epub 2024 Dec 10. Comput Biol Med. 2025. PMID: 39662317
-
Microsecond and nanosecond electric pulses in cancer treatments.Bioelectromagnetics. 2012 Feb;33(2):106-23. doi: 10.1002/bem.20692. Epub 2011 Aug 3. Bioelectromagnetics. 2012. PMID: 21812011 Review.
-
Electrochemotherapy as a New Modality in Interventional Oncology: A Review.Technol Cancer Res Treat. 2018 Jan 1;17:1533033818785329. doi: 10.1177/1533033818785329. Technol Cancer Res Treat. 2018. PMID: 29986632 Free PMC article. Review.
Cited by
-
In-silico tool based on Boolean networks and meshless simulations for prediction of reaction and transport mechanisms in the systemic administration of chemotherapeutic drugs.PLoS One. 2025 Feb 7;20(2):e0315194. doi: 10.1371/journal.pone.0315194. eCollection 2025. PLoS One. 2025. PMID: 39919263 Free PMC article.
References
-
- Zhan W. 2014. Mathematical Modelling of Drug Delivery to Solid Tumour.
-
- Fuso Nerini I., Morosi L., et al. D’Incalci M. Intratumor heterogeneity and its impact on drug distribution and sensitivity. Clin. Pharmacol. Ther. 2014;96:224–238. - PubMed
-
- Tannock I.F., Lee C.M., et al. Egorin M.J. Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin. Cancer Res. 2002;8:878–884. - PubMed
-
- Batista Napotnik T., Miklavčič D. In vitro electroporation detection methods – An overview. Bioelectrochemistry. 2018;120:166–182. https://www.sciencedirect.com/science/article/pii/S156753941730395X?via%... - PubMed
-
- Kotnik T., Rems L., et al. Miklavčič D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019;48:63–91. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

