TACI and endogenous APRIL in B cell maturation
- PMID: 37422057
- PMCID: PMC10528899
- DOI: 10.1016/j.clim.2023.109689
TACI and endogenous APRIL in B cell maturation
Abstract
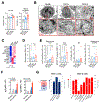
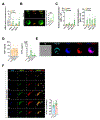
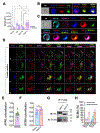
While many of the genes and molecular pathways in the germinal center B cell response which initiate protective antibody production are known, the contributions of individual molecular players in terminal B cell differentiation remain unclear. We have previously investigated how mutations in TACI gene, noted in about 10% of patients with common variable immunodeficiency, impair B cell differentiation and often, lead to lymphoid hyperplasia and autoimmunity. Unlike mouse B cells, human B cells express TACI-L (Long) and TACI-S (Short) isoforms, but only TACI-S promotes terminal B cell differentiation into plasma cells. Here we show that the expression of intracellular TACI-S increases with B cell activation, and colocalizes with BCMA and their ligand, APRIL. We show that the loss of APRIL impairs isotype class switch and leads to distinct metabolic and transcriptional changes. Our studies suggest that intracellular TACI-S and APRIL along with BCMA direct long-term PC differentiation and survival.
Keywords: A proliferation inducing ligand (APRIL); BCMA (B cell maturation antigen); Plasma cell; TACI (transmembrane activator and CAML interactor).
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Authors declare that they have no competing interests to disclosure.
Figures

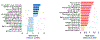

Similar articles
-
Unique and redundant roles of mouse BCMA, TACI, BAFF, APRIL, and IL-6 in supporting antibody-producing cells in different tissues.Proc Natl Acad Sci U S A. 2024 Jul 16;121(29):e2404309121. doi: 10.1073/pnas.2404309121. Epub 2024 Jul 11. Proc Natl Acad Sci U S A. 2024. PMID: 38990948 Free PMC article.
-
TACI-dependent APRIL signaling maintains autoreactive B cells in a mouse model of systemic lupus erythematosus.Eur J Immunol. 2017 Apr;47(4):713-723. doi: 10.1002/eji.201646630. Eur J Immunol. 2017. PMID: 28267197
-
Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL.Blood. 2007 Jan 15;109(2):729-39. doi: 10.1182/blood-2006-04-015958. Epub 2006 Sep 7. Blood. 2007. PMID: 16960154 Free PMC article.
-
Transmembrane activator and calcium-modulator and cyclophilin ligand interactor mutations in common variable immunodeficiency.Curr Opin Allergy Clin Immunol. 2008 Dec;8(6):520-6. doi: 10.1097/ACI.0b013e3283141200. Curr Opin Allergy Clin Immunol. 2008. PMID: 18978466 Review.
-
TACItly changing tunes: farewell to a yin and yang of BAFF receptor and TACI in humoral immunity? New genetic defects in common variable immunodeficiency.Curr Opin Allergy Clin Immunol. 2005 Dec;5(6):496-503. doi: 10.1097/01.all.0000191887.89773.cc. Curr Opin Allergy Clin Immunol. 2005. PMID: 16264328 Review.
Cited by
-
Efficacy and safety of telitacicept in patients with systemic lupus erythematosus: a multicentre, retrospective, real-world study.Lupus Sci Med. 2023 Nov 24;10(2):e001074. doi: 10.1136/lupus-2023-001074. Lupus Sci Med. 2023. PMID: 38007228 Free PMC article.
-
Combination treatment with telitacicept, cyclophosphamide and glucocorticoids for severe Granulomatous polyangiitis: a case report and literature review.Front Immunol. 2023 Dec 1;14:1298650. doi: 10.3389/fimmu.2023.1298650. eCollection 2023. Front Immunol. 2023. PMID: 38106422 Free PMC article. Review.
-
Drugs in Development to Treat IgA Nephropathy.Drugs. 2024 May;84(5):503-525. doi: 10.1007/s40265-024-02036-1. Epub 2024 May 23. Drugs. 2024. PMID: 38777962 Review.
-
Pathogenic TNFRSF13B Variant in an Adult Japanese Patient with Common Variable Immunodeficiency.Intern Med. 2025 Mar 1;64(5):753-757. doi: 10.2169/internalmedicine.4057-24. Epub 2024 Jul 11. Intern Med. 2025. PMID: 38987180 Free PMC article.
-
B Cell Activating Factor Induces Drug Resistance in Hairy Cell Leukemia Variant.Biomedicines. 2025 Apr 7;13(4):890. doi: 10.3390/biomedicines13040890. Biomedicines. 2025. PMID: 40299479 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

