Aberrations in Energetic Metabolism and Stress-Related Pathways Contribute to Pathophysiology in the Neb Conditional Knockout Mouse Model of Nemaline Myopathy
- PMID: 37422147
- PMCID: PMC10548278
- DOI: 10.1016/j.ajpath.2023.06.009
Aberrations in Energetic Metabolism and Stress-Related Pathways Contribute to Pathophysiology in the Neb Conditional Knockout Mouse Model of Nemaline Myopathy
Abstract
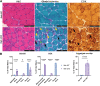
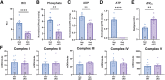
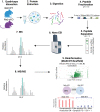
Nemaline myopathy (NM) is a genetically and clinically heterogeneous disease that is diagnosed on the basis of the presence of nemaline rods on skeletal muscle biopsy. Although NM has typically been classified by causative genes, disease severity or prognosis cannot be predicted. The common pathologic end point of nemaline rods (despite diverse genetic causes) and an unexplained range of muscle weakness suggest that shared secondary processes contribute to the pathogenesis of NM. We speculated that these processes could be identified through a proteome-wide interrogation using a mouse model of severe NM in combination with pathway validation and structural/functional analyses. A proteomic analysis was performed using skeletal muscle tissue from the Neb conditional knockout mouse model compared with its wild-type counterpart to identify pathophysiologically relevant biological processes that might impact disease severity or provide new treatment targets. A differential expression analysis and Ingenuity Pathway Core Analysis predicted perturbations in several cellular processes, including mitochondrial dysfunction and changes in energetic metabolism and stress-related pathways. Subsequent structural and functional studies demonstrated abnormal mitochondrial distribution, decreased mitochondrial respiratory function, an increase in mitochondrial transmembrane potential, and extremely low ATP content in Neb conditional knockout muscles relative to wild type. Overall, the findings of these studies support a role for severe mitochondrial dysfunction as a novel contributor to muscle weakness in NM.
Copyright © 2023 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures







Similar articles
-
ACTA1 H40Y mutant iPSC-derived skeletal myocytes display mitochondrial defects in an in vitro model of nemaline myopathy.Exp Cell Res. 2023 Mar 15;424(2):113507. doi: 10.1016/j.yexcr.2023.113507. Epub 2023 Feb 14. Exp Cell Res. 2023. PMID: 36796746 Free PMC article.
-
Myosin ATPase inhibition fails to rescue the metabolically dysregulated proteome of nebulin-deficient muscle.J Physiol. 2024 Oct;602(20):5229-5245. doi: 10.1113/JP286870. Epub 2024 Aug 31. J Physiol. 2024. PMID: 39216086
-
Deleting exon 55 from the nebulin gene induces severe muscle weakness in a mouse model for nemaline myopathy.Brain. 2013 Jun;136(Pt 6):1718-31. doi: 10.1093/brain/awt113. Epub 2013 May 28. Brain. 2013. PMID: 23715096 Free PMC article.
-
Recent advances in nemaline myopathy.Neuromuscul Disord. 2021 Oct;31(10):955-967. doi: 10.1016/j.nmd.2021.07.012. Epub 2021 Jul 24. Neuromuscul Disord. 2021. PMID: 34561123 Review.
-
Nemaline myopathies: a current view.J Muscle Res Cell Motil. 2019 Jun;40(2):111-126. doi: 10.1007/s10974-019-09519-9. Epub 2019 Jun 21. J Muscle Res Cell Motil. 2019. PMID: 31228046 Free PMC article. Review.
Cited by
-
Integrated single-cell functional-proteomic profiling reveals a shift in myofibre specificity in human nemaline myopathy: A proof-of-principle study.J Physiol. 2025 May;603(10):3033-3048. doi: 10.1113/JP288363. Epub 2025 May 5. J Physiol. 2025. PMID: 40320980 Free PMC article.
-
Signatures of Cysteine Oxidation on Muscle Structural and Contractile Proteins Are Associated with Physical Performance and Muscle Function in Older Adults: Study of Muscle, Mobility and Aging (SOMMA).medRxiv [Preprint]. 2023 Nov 8:2023.11.07.23298224. doi: 10.1101/2023.11.07.23298224. medRxiv. 2023. Update in: Aging Cell. 2024 Jun;23(6):e14094. doi: 10.1111/acel.14094. PMID: 37986748 Free PMC article. Updated. Preprint.
-
Actin Polymerization Defects Induce Mitochondrial Dysfunction in Cellular Models of Nemaline Myopathies.Antioxidants (Basel). 2023 Nov 21;12(12):2023. doi: 10.3390/antiox12122023. Antioxidants (Basel). 2023. PMID: 38136143 Free PMC article.
-
Different Mouse Models of Nemaline Myopathy Harboring Acta1 Mutations Display Differing Abnormalities Related to Mitochondrial Biology.Am J Pathol. 2023 Oct;193(10):1548-1567. doi: 10.1016/j.ajpath.2023.06.008. Epub 2023 Jul 5. Am J Pathol. 2023. PMID: 37419385 Free PMC article.
-
Signatures of cysteine oxidation on muscle structural and contractile proteins are associated with physical performance and muscle function in older adults: Study of Muscle, Mobility and Aging (SOMMA).Aging Cell. 2024 Jun;23(6):e14094. doi: 10.1111/acel.14094. Epub 2024 Feb 8. Aging Cell. 2024. PMID: 38332629 Free PMC article.
References
-
- Kondo E., Nishimura T., Kosho T., Inaba Y., Mitsuhashi S., Ishida T., Baba A., Koike K., Nishino I., Nonaka I., Furukawa T., Saito K. Recessive RYR1 mutations in a patient with severe congenital nemaline myopathy with ophthalomoplegia identified through massively parallel sequencing. Am J Med Genet A. 2012;158a:772–778. - PubMed
-
- Pelin K., Donner K., Holmberg M., Jungbluth H., Muntoni F., Wallgren-Pettersson C. Nebulin mutations in autosomal recessive nemaline myopathy: an update. Neuromuscul Disord. 2002;12:680–686. - PubMed
-
- Pelin K., Hilpela P., Donner K., Sewry C., Akkari P.A., Wilton S.D., Wattanasirichaigoon D., Bang M.L., Centner T., Hanefeld F., Odent S., Fardeau M., Urtizberea J.A., Muntoni F., Dubowitz V., Beggs A.H., Laing N.G., Labeit S., de la Chapelle A., Wallgren-Pettersson C. Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci U S A. 1999;96:2305–2310. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

