Study of the biosynthesis and functionality of polyphosphate in Bifidobacterium longum KABP042
- PMID: 37422465
- PMCID: PMC10329679
- DOI: 10.1038/s41598-023-38082-0
Study of the biosynthesis and functionality of polyphosphate in Bifidobacterium longum KABP042
Abstract
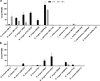
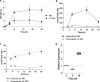
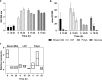
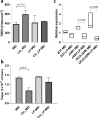
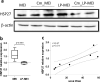
Polyphosphate (poly-P) biosynthesis in bacteria has been linked to many physiological processes and has been characterized as an interesting functional molecule involved in intestinal homeostasis. We determined the capacity for poly-P production of 18 probiotic strains mainly belonging to Bifidobacterium and former Lactobacillus genera, showing that poly-P synthesis varied widely between strains and is dependent on the availability of phosphate and the growth phase. Bifidobacteria were especially capable of poly-P synthesis and poly-P kinase (ppk) genes were identified in their genomes together with a repertoire of genes involved in phosphate transport and metabolism. In Bifidobacterium longum KABP042, the strain we found with highest poly-P production, variations in ppk expression were linked to growth conditions and presence of phosphate in the medium. Moreover, the strain produced poly-P in presence of breast milk and lacto-N-tetraose increased the amount of poly-P synthesized. Compared to KABP042 supernatants low in poly-P, exposure of Caco-2 cells to KABP042 supernatants rich in poly-P resulted in decreased epithelial permeability and increased barrier resistance, induction of epithelial protecting factors such as HSP27 and enhanced expression of tight junction protein genes. These results highlight the role of bifidobacteria-derived poly-P as a strain-dependent functional factor acting on epithelial integrity.
© 2023. The Author(s).
Conflict of interest statement
PH, TA, MP and JE-M are employees of AB-Biotics S.A. (part of KANEKA Corporation). The isolates described in this study are the subject of a pending patent application co-authored by MP and JE-M. The remaining authors have no conflict of interest to declare.
Figures

Similar articles
-
Probiotic Properties of Bifidobacterium longum KABP042 and Pediococcus pentosaceus KABP041 Show Potential to Counteract Functional Gastrointestinal Disorders in an Observational Pilot Trial in Infants.Front Microbiol. 2022 Jan 12;12:741391. doi: 10.3389/fmicb.2021.741391. eCollection 2021. Front Microbiol. 2022. PMID: 35095783 Free PMC article.
-
Lactobacillus acidophilus and Bifidobacterium longum supernatants upregulate the serotonin transporter expression in intestinal epithelial cells.Saudi J Gastroenterol. 2018 Jan-Feb;24(1):59-66. doi: 10.4103/sjg.SJG_333_17. Saudi J Gastroenterol. 2018. PMID: 29451186 Free PMC article.
-
Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway.PLoS One. 2011;6(8):e23278. doi: 10.1371/journal.pone.0023278. Epub 2011 Aug 15. PLoS One. 2011. PMID: 21858054 Free PMC article.
-
Bosom Buddies: The Symbiotic Relationship Between Infants and Bifidobacterium longum ssp. longum and ssp. infantis. Genetic and Probiotic Features.Annu Rev Food Sci Technol. 2016;7:1-21. doi: 10.1146/annurev-food-041715-033151. Annu Rev Food Sci Technol. 2016. PMID: 26934170 Review.
-
Bifidobacterium longum W11: Uniqueness and individual or combined clinical use in association with rifaximin.Clin Nutr ESPEN. 2021 Apr;42:15-21. doi: 10.1016/j.clnesp.2020.12.025. Epub 2021 Feb 17. Clin Nutr ESPEN. 2021. PMID: 33745570 Review.
Cited by
-
Lactobacillus plantarum-Derived Inorganic Polyphosphate Regulates Immune Function via Inhibiting M1 Polarization and Resisting Oxidative Stress in Macrophages.Antioxidants (Basel). 2025 Apr 1;14(4):428. doi: 10.3390/antiox14040428. Antioxidants (Basel). 2025. PMID: 40298816 Free PMC article.
-
Effects of p-coumaric acid on probiotic properties of Lactobacillus acidophilus LA-5 and lacticaseibacillus rhamnosus GG.Arch Microbiol. 2024 Apr 20;206(5):223. doi: 10.1007/s00203-024-03957-x. Arch Microbiol. 2024. PMID: 38642150
-
Polyphosphate from Lactic Acid Bacteria: A Functional Molecule for Food and Health Applications.Foods. 2025 Jun 23;14(13):2211. doi: 10.3390/foods14132211. Foods. 2025. PMID: 40646963 Free PMC article. Review.
-
Inorganic phosphate modifies stationary phase fitness and metabolic pathways in Lactiplantibacillus paraplantarum CRL 1905.Front Microbiol. 2024 Feb 27;15:1343541. doi: 10.3389/fmicb.2024.1343541. eCollection 2024. Front Microbiol. 2024. PMID: 38476941 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

