SCP2 mediates the transport of lipid hydroperoxides to mitochondria in chondrocyte ferroptosis
- PMID: 37422468
- PMCID: PMC10329676
- DOI: 10.1038/s41420-023-01522-x
SCP2 mediates the transport of lipid hydroperoxides to mitochondria in chondrocyte ferroptosis
Abstract
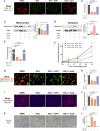
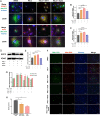
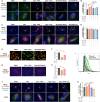
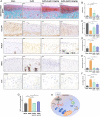
Sterol carrier protein 2 (SCP2) is highly expressed in human osteoarthritis (OA) cartilage, accompanied by ferroptosis hallmarks, especially the accumulation of lipid hydroperoxides (LPO). However, the role of SCP2 in chondrocyte ferroptosis remains unexplored. Here, we identify that SCP2 transports cytoplasmic LPO to mitochondria in RSL3-induced chondrocyte ferroptosis, resulting in mitochondrial membrane damage and release of reactive oxygen species (ROS). The localization of SCP2 on mitochondria is associated with mitochondrial membrane potential, but independent of microtubules transport or voltage-dependent anion channel. Moreover, SCP2 promotes lysosomal LPO increase and lysosomal membrane damage through elevating ROS. However, SCP2 is not directly involved in the cell membrane rupture caused by RSL3. Inhibition of SCP2 markedly protects mitochondria and reduces LPO levels, attenuating chondrocyte ferroptosis in vitro and alleviating the progression of OA in rats. Our study demonstrates that SCP2 mediates the transport of cytoplasmic LPO to mitochondria and the spread of intracellular LPO, accelerating chondrocyte ferroptosis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Theaflavin-3,3'-Digallate Inhibits Erastin-Induced Chondrocytes Ferroptosis via the Nrf2/GPX4 Signaling Pathway in Osteoarthritis.Oxid Med Cell Longev. 2022 Nov 17;2022:3531995. doi: 10.1155/2022/3531995. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36439689 Free PMC article.
-
Astaxanthin attenuates osteoarthritis progression via inhibiting ferroptosis and regulating mitochondrial function in chondrocytes.Chem Biol Interact. 2022 Oct 1;366:110148. doi: 10.1016/j.cbi.2022.110148. Epub 2022 Sep 7. Chem Biol Interact. 2022. PMID: 36084724
-
Sterol carrier and lipid transfer proteins.Chem Phys Lipids. 1985 Sep;38(3):239-61. doi: 10.1016/0009-3084(85)90019-2. Chem Phys Lipids. 1985. PMID: 3910286 Review.
-
Sterol Carrier Protein-2, a Nonspecific Lipid-Transfer Protein, in Intracellular Cholesterol Trafficking in Testicular Leydig Cells.PLoS One. 2016 Feb 22;11(2):e0149728. doi: 10.1371/journal.pone.0149728. eCollection 2016. PLoS One. 2016. PMID: 26901662 Free PMC article.
-
Ferroptosis in Osteoarthritis: Current Understanding.J Inflamm Res. 2024 Nov 7;17:8471-8486. doi: 10.2147/JIR.S493001. eCollection 2024. J Inflamm Res. 2024. PMID: 39529997 Free PMC article. Review.
Cited by
-
Quercetin attenuates the symptoms of osteoarthritis in vitro and in vivo by suppressing ferroptosis via activation of AMPK/Nrf2/Gpx4 signaling.Mol Med Rep. 2025 Mar;31(3):60. doi: 10.3892/mmr.2024.13425. Epub 2024 Dec 24. Mol Med Rep. 2025. PMID: 39717946 Free PMC article.
-
Combining Multiple Omics with Molecular Dynamics Reveals SCP2-Mediated Cytotoxicity Effects of Aflatoxin B1 in SW480 Cells.Toxins (Basel). 2024 Aug 24;16(9):375. doi: 10.3390/toxins16090375. Toxins (Basel). 2024. PMID: 39330833 Free PMC article.
-
[Multi-omics analysis of the relationship between oxidative stress-related gene and prostate cancer].Beijing Da Xue Xue Bao Yi Xue Ban. 2025 Aug 18;57(4):633-643. doi: 10.19723/j.issn.1671-167X.2025.04.002. Beijing Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40754897 Free PMC article. Chinese.
-
Iron metabolism and arthritis: Exploring connections and therapeutic avenues.Chin Med J (Engl). 2024 Jul 20;137(14):1651-1662. doi: 10.1097/CM9.0000000000003169. Epub 2024 Jun 12. Chin Med J (Engl). 2024. PMID: 38867424 Free PMC article. Review.
-
New Targets for Immune Inflammatory Response in Rheumatoid Arthritis: Focus on the Potential Significance of N6-Methyladenosine, Ferroptosis and Cuproptosis.J Inflamm Res. 2025 Jun 19;18:8085-8106. doi: 10.2147/JIR.S526096. eCollection 2025. J Inflamm Res. 2025. PMID: 40551986 Free PMC article. Review.
References
-
- Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. The Lancet (British edition) 2019;393:1745–59.. - PubMed
LinkOut - more resources
Full Text Sources

