Ketones from aldehydes via alkyl C(sp3)-H functionalization under photoredox cooperative NHC/palladium catalysis
- PMID: 37422483
- PMCID: PMC10329650
- DOI: 10.1038/s41467-023-39707-8
Ketones from aldehydes via alkyl C(sp3)-H functionalization under photoredox cooperative NHC/palladium catalysis
Abstract
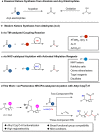
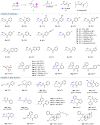
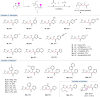
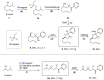
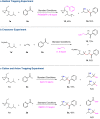
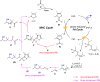
Direct synthesis of ketones from aldehydes features high atom- and step-economy. Yet, the coupling of aldehydes with unactivated alkyl C(sp3)-H remains challenging. Herein, we develop the synthesis of ketones from aldehydes via alkyl C(sp3)-H functionalization under photoredox cooperative NHC/Pd catalysis. The two-component reaction of iodomethylsilyl alkyl ether with aldehydes gave a variety of β-, γ- and δ-silyloxylketones via 1,n-HAT (n = 5, 6, 7) of silylmethyl radicals to generate secondary or tertiary alkyl radicals and following coupling with ketyl radicals from aldehydes under photoredox NHC catalysis. The three-component reaction with the addition of styrenes gave the corresponding ε-hydroxylketones via the generation of benzylic radicals by the addition of alkyl radicals to styrenes and following coupling with ketyl radicals. This work demonstrates the generation of ketyl radical and alkyl radical under the photoredox cooperative NHC/Pd catalysis, and provides two and three component reactions for the synthesis of ketones from aldehydes with alkyl C(sp3)-H functionalization. The synthetic potential of this protocol was also further illustrated by the late-stage functionalization of natural products.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Cooperative NHC and Photoredox Catalysis for the Synthesis of β-Trifluoromethylated Alkyl Aryl Ketones.Angew Chem Int Ed Engl. 2020 Nov 2;59(45):19956-19960. doi: 10.1002/anie.202008040. Epub 2020 Sep 1. Angew Chem Int Ed Engl. 2020. PMID: 32700458 Free PMC article.
-
Synthetic and Mechanistic Implications of Chlorine Photoelimination in Nickel/Photoredox C(sp3)-H Cross-Coupling.Acc Chem Res. 2021 Feb 16;54(4):988-1000. doi: 10.1021/acs.accounts.0c00694. Epub 2021 Jan 29. Acc Chem Res. 2021. PMID: 33511841 Free PMC article.
-
Photoredox cooperative N-heterocyclic carbene/palladium-catalysed alkylacylation of alkenes.Nat Commun. 2022 Sep 30;13(1):5754. doi: 10.1038/s41467-022-33444-0. Nat Commun. 2022. PMID: 36180483 Free PMC article.
-
Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development.ACS Catal. 2017 Apr 7;7(4):2563-2575. doi: 10.1021/acscatal.7b00094. Epub 2017 Mar 14. ACS Catal. 2017. PMID: 28413692 Free PMC article. Review.
-
Palladium(0)-Catalyzed Benzylic C(sp3)-H Functionalization for the Concise Synthesis of Heterocycles and Its Applications.Chem Pharm Bull (Tokyo). 2017;65(5):409-425. doi: 10.1248/cpb.c16-00969. Chem Pharm Bull (Tokyo). 2017. PMID: 28458363 Review.
Cited by
-
N-Heterocyclic carbene catalytic 1,2-boron migrative acylation accelerated by photocatalysis.Sci Adv. 2024 Jul 26;10(30):eadn8401. doi: 10.1126/sciadv.adn8401. Epub 2024 Jul 24. Sci Adv. 2024. PMID: 39047096 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

