Adverse effects of 21 antidepressants on sleep during acute-phase treatment in major depressive disorder: a systemic review and dose-effect network meta-analysis
- PMID: 37422714
- PMCID: PMC10566234
- DOI: 10.1093/sleep/zsad177
Adverse effects of 21 antidepressants on sleep during acute-phase treatment in major depressive disorder: a systemic review and dose-effect network meta-analysis
Abstract
Study objectives: Sleep-related adverse effects during acute treatment with antidepressants undermine adherence and impede remission. We aimed to address subtypes of sleep-related adverse effects and depict the relationship between dose and sleep-related adverse events.
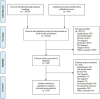
Methods: We searched PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science for double-blind randomized controlled trials of depression published before April 30th, 2023. Eligible studies reporting sleep-related adverse effects during short-term monotherapy were included. The odds ratios (ORs) for sleep-related adverse effects were addressed with network meta-analysis. A Bayesian approach was used to depict the dose-effect relationship. Heterogeneity among studies was assessed using the τ2 and I2 statistics. Sensitivity analyses were performed without studies featuring high risk of bias.
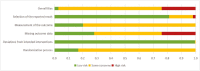
Results: Studies with 64 696 patients were examined from 216 trials. Compared to placebo, 13 antidepressants showed higher ORs for somnolence, of which fluvoxamine (OR = 6.32; 95% CI: 3.56 to 11.21) ranked the top. Eleven had higher risks for insomnia, reboxetine ranked the top (OR = 3.47; 95% CI: 2.77 to 4.36). The dose-effect relationships curves between somnolence or insomnia and dose included linear shape, inverted U-shape, and other shapes. There was no significant heterogeneity among individual studies. The quality of evidence for results in network meta-analyses was rated as very low to moderate by Grading of Recommendations Assessment, Development, and Evaluation.
Conclusions: Most antidepressants had higher risks for insomnia or somnolence than placebo. The diverse relationship curves between somnolence or insomnia and dose of antidepressants can guide clinicians to adjust the doses. These findings suggest clinicians pay more attention to sleep-related adverse effects during acute treatment with antidepressants.
Keywords: adverse effects; antidepressants; insomnia; major depressive disorders; somnolence.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Sleep Research Society.
Figures



Similar articles
-
Antidepressants plus benzodiazepines for adults with major depression.Cochrane Database Syst Rev. 2019 Jun 3;6(6):CD001026. doi: 10.1002/14651858.CD001026.pub2. Cochrane Database Syst Rev. 2019. PMID: 31158298 Free PMC article.
-
Antidepressants for people with epilepsy and depression.Cochrane Database Syst Rev. 2021 Apr 16;4(4):CD010682. doi: 10.1002/14651858.CD010682.pub3. Cochrane Database Syst Rev. 2021. PMID: 33860531 Free PMC article.
-
Insomnia and somnolence associated with second-generation antidepressants during the treatment of major depression: a meta-analysis.J Clin Psychopharmacol. 2015 Jun;35(3):296-303. doi: 10.1097/JCP.0000000000000329. J Clin Psychopharmacol. 2015. PMID: 25874915
-
Clinical interventions for adults with comorbid alcohol use and depressive disorders: A systematic review and network meta-analysis.PLoS Med. 2021 Oct 8;18(10):e1003822. doi: 10.1371/journal.pmed.1003822. eCollection 2021 Oct. PLoS Med. 2021. PMID: 34624018 Free PMC article.
-
Eszopiclone for insomnia.Cochrane Database Syst Rev. 2018 Oct 10;10(10):CD010703. doi: 10.1002/14651858.CD010703.pub2. Cochrane Database Syst Rev. 2018. PMID: 30303519 Free PMC article.
Cited by
-
Effectiveness of Vortioxetine for the Treatment of Emotional Blunting in Patients with Major Depressive Disorder Experiencing Inadequate Response to SSRI/SNRI Monotherapy in Spain: Results from the COMPLETE Study.Neuropsychiatr Dis Treat. 2024 Jul 30;20:1475-1489. doi: 10.2147/NDT.S473056. eCollection 2024. Neuropsychiatr Dis Treat. 2024. PMID: 39100571 Free PMC article.
-
Association between polypharmacy and the long-term prescription of hypnotics in Japan: a retrospective cross-sectional study.Front Psychiatry. 2024 Dec 9;15:1471457. doi: 10.3389/fpsyt.2024.1471457. eCollection 2024. Front Psychiatry. 2024. PMID: 39717375 Free PMC article.
-
Pharmacological Monotherapy for Depressive Disorders: Current and Future-A Narrative Review.Medicina (Kaunas). 2025 Mar 21;61(4):558. doi: 10.3390/medicina61040558. Medicina (Kaunas). 2025. PMID: 40282849 Free PMC article. Review.
-
Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora.Biomedicines. 2025 May 7;13(5):1129. doi: 10.3390/biomedicines13051129. Biomedicines. 2025. PMID: 40426956 Free PMC article. Review.
References
-
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

