AgRP neurons are not indispensable for body weight maintenance in adult mice
- PMID: 37422762
- PMCID: PMC10909125
- DOI: 10.1016/j.celrep.2023.112789
AgRP neurons are not indispensable for body weight maintenance in adult mice
Abstract
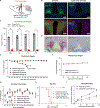
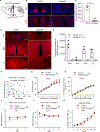
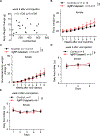
In addition to their role in promoting feeding and obesity development, hypothalamic arcuate agouti-related protein/neuropeptide Y (AgRP/NPY) neurons are widely perceived to be indispensable for maintaining normal feeding and body weight in adults, and consistently, acute inhibition of AgRP neurons is known to reduce short-term food intake. Here, we adopted complementary methods to achieve nearly complete ablation of arcuate AgRP/NPY neurons in adult mice and report that lesioning arcuate AgRP/NPY neurons in adult mice causes no apparent alterations in ad libitum feeding or body weight. Consistent with previous studies, loss of AgRP/NPY neurons blunts fasting refeeding. Thus, our studies show that AgRP/NPY neurons are not required for maintaining ad libitum feeding or body weight homeostasis in adult mice.
Keywords: CP: Neuroscience.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

