Cortical regulation of neurogenesis and cell proliferation in the ventral subventricular zone
- PMID: 37422764
- PMCID: PMC10422956
- DOI: 10.1016/j.celrep.2023.112783
Cortical regulation of neurogenesis and cell proliferation in the ventral subventricular zone
Abstract
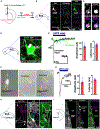
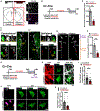
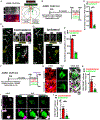
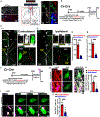
Neurogenesis and differentiation of neural stem cells (NSCs) are controlled by cell-intrinsic molecular pathways that interact with extrinsic signaling cues. In this study, we identify a circuit that regulates neurogenesis and cell proliferation in the lateral ventricle-subventricular zone (LV-SVZ). Our results demonstrate that direct glutamatergic projections from the anterior cingulate cortex (ACC), as well as inhibitory projections from calretinin+ local interneurons, modulate the activity of cholinergic neurons in the subependymal zone (subep-ChAT+). Furthermore, in vivo optogenetic stimulation and inhibition of the ACC-subep-ChAT+ circuit are sufficient to control neurogenesis in the ventral SVZ. Both subep-ChAT+ and local calretinin+ neurons play critical roles in regulating ventral SVZ neurogenesis and LV-SVZ cell proliferation.
Keywords: CP: Neuroscience; anterior cingulate; calretinin; frontal cortex; neural stem cells proliferation; postnatal neurogenesis; subep-ChAT(+); subventricular zone.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Similar articles
-
Regulation of neural stem cells by innervating neurons.J Neurochem. 2025 Jan;169(1):e16287. doi: 10.1111/jnc.16287. J Neurochem. 2025. PMID: 39775528 Free PMC article. Review.
-
Lateral Ventricular Neural Stem Cells Provide Negative Feedback to Circuit Activation Through GABAergic Signaling.Cells. 2025 Mar 13;14(6):426. doi: 10.3390/cells14060426. Cells. 2025. PMID: 40136675 Free PMC article.
-
A cholinergic signaling pathway underlying cortical circuit activation of quiescent neural stem cells in the lateral ventricle.Sci Signal. 2024 Sep 24;17(855):eadk8810. doi: 10.1126/scisignal.adk8810. Epub 2024 Sep 24. Sci Signal. 2024. PMID: 39316665
-
ChAT-positive neurons participate in subventricular zone neurogenesis after middle cerebral artery occlusion in mice.Behav Brain Res. 2017 Jan 1;316:145-151. doi: 10.1016/j.bbr.2016.09.007. Epub 2016 Sep 5. Behav Brain Res. 2017. PMID: 27609645 Free PMC article.
-
The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis.Cold Spring Harb Perspect Biol. 2016 May 2;8(5):a018820. doi: 10.1101/cshperspect.a018820. Cold Spring Harb Perspect Biol. 2016. PMID: 27048191 Free PMC article. Review.
Cited by
-
Neurogenesis dynamics in the olfactory bulb: deciphering circuitry organization, function, and adaptive plasticity.Neural Regen Res. 2025 Jun 1;20(6):1565-1581. doi: 10.4103/NRR.NRR-D-24-00312. Epub 2024 Jun 26. Neural Regen Res. 2025. PMID: 38934393 Free PMC article.
-
Regulation of neural stem cells by innervating neurons.J Neurochem. 2025 Jan;169(1):e16287. doi: 10.1111/jnc.16287. J Neurochem. 2025. PMID: 39775528 Free PMC article. Review.
-
Lateral Ventricular Neural Stem Cells Provide Negative Feedback to Circuit Activation Through GABAergic Signaling.Cells. 2025 Mar 13;14(6):426. doi: 10.3390/cells14060426. Cells. 2025. PMID: 40136675 Free PMC article.
References
-
- Obernier K, Cebrian-Silla A,Thomson M, Parraguez JI, Anderson R, Guinto C, Rodas Rodriguez J, Garcia-Verdugo JM, and Alvarez-Buylla A (2018). Adult Neurogenesis Is Sustained by Symmetric Self-Renewal and Differentiation. Cell Stem Cell 22, 221–234.e8. 10.1016/j.stem.2018.01.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

